AbstractHighly-intensive exercise occurs in reactive oxygen species which leads to cellular damage as a result of increased oxidative stress markers. An appropriate program design incorporating volume, intensity, and types of exercise may produce different effects amid oxidative status in athletes. Therefore, this study aimed to investigate the outcomes of resistance training (RT) and high-intensity interval training (HIIT) on oxidative stress markers, and physical performance in university athlete subjects. The effect of two different protocol types (RT and HIIT) on oxidative stress and antioxidant status were also compared. Seventy-two university athletes, were recruited and divided into control, RT, and HIIT groups (n=22/group). The RT group were undertaken a resistance exercise training program of the upper and lower body. The HIIT group performed their exercise training on a cycle ergometer. The training program was 30 min/day, 3 days/wk, continuously over 8 weeks. We observed that long-term RT and HIIT improved blood glutathione and glutathione disulfide redox ratio in all athletes. The results demonstrated that only RT training significantly decreased plasma malondialdehyde. Another finding was that RT and HIIT resulted in similar elicitation of physical performance in the post-compared with pretraining exercise. This study revealed that RT and HIIT programs improved antioxidants and physical performance in university sports athletes. However, oxidative markers were only improved following the RT program. This study suggests that RT program is superior to HIIT in improving oxidative stress markers in sport athletes.
INTRODUCTIONVery intensive exercise happens in reactive oxygen species (ROS) which leads to cellular damage resultant of increased oxidative stress markers (Finaud et al., 2006; Jurgenson et al., 2019). The major intracellular source of reactive oxygen in species is damaged mitochondria of the muscle cells (Finaud et al., 2006). It is well known that skeletal muscles produce significantly increased amounts of superoxide anion in a single bout of exercise (Mastaloudis et al., 2004).
Resistance training (RT) is an essential component of training for health, fitness, and sports performance. The effects of RT are associated with greater strength and power in sport athletes. Moderate to high RT training improvements to oxidative stress status have been published by several researchers (Azizbeigi et al., 2013; Azizbeigi et al., 2015; Parise et al., 2005; Ryrso et al., 2018). However, a high volume of RT did not change oxidative stress markers, nor antioxidants in healthy subjects (Koozehchian et al., 2018). Furthermore, high-intensity strength training increases oxidative stress status and decreases total antioxidant capacity in well-trained power-lifting athletes (Jurgenson et al., 2019). Nevertheless, this may lead to the unfavorable effects of high-volume exercise on health outcomes.
High-intensity interval training (HIIT) is a type of aerobic exercise frequently used by endurance athletes as a method of eliciting cardiovascular and metabolic performances (Billat, 2001; Buchheit et al., 2010; Gist et al., 2015; Menz et al., 2019). Our recent data suggest that short durations of high-intensity exercise interspersed with a low cycle ergometer volume was capable of inducing favorable training adaptations such as improved body composition, nerve conduction amplitude, and heart rate variability (Padkao and Prasertsri, 2019). Short-term HIIT has also been reported to promote antioxidant activity and attenuate oxidative stress in healthy women (Bogdanis et al., 2013). Moreover, long-term HIIT reduced oxidative stress in the aged rat model (Li et al., 2018). In addition, regular aerobic exercise with optimal load has been shown to improve antioxidant adaptation to exercise (Bloomer et al., 2006; Higgins et al., 2020). Nonetheless, previous studies demonstrated that both RT and aerobic training did not alter oxidative stress adaptations in athletes (Park and Kwak, 2016). It is interesting to note that those protocols performed well in terms of improving oxidative stress markers, yet outcomes were not greatly improved. Thus, an appropriate program design incorporating volume, intensity, and types of exercise may produce different effects on oxidative status in athletes. Consequently, the optimum amount of volume and intensity in the RT program should obtain positive data on oxidative stress markers.
Based on our previous study, a RT program was designed in such a way to render efforts in moving high resistance loading at 70% of one-repetition maximum (1RM) interspersed with low resistance loading at 30% of 1RM (Padkao and Prasertsri, 2019). The results of that study found RT incorporating upper and lower limb muscles improved motor nerve conduction amplitude and body composition. Besides, HIIT training was improved cardiac autonomic activity in athletes. Regarding this, the effect of RT and HIIT on oxidative stress and antioxidant status has not yet been reported, it is relevant to identify what would be the actual effect of these protocols on oxidative stress and antioxidant status.
Therefore, the aim of this study was to investigate outcomes of RT and HIIT on oxidative stress markers as well as physical performance in university athlete subjects. The effect of two different protocol types (RT and HIIT) on oxidative stress and antioxidant status were also compared.
MATERIALS AND METHODSParticipants and experimental designFollowing ethical approval from the Burapha University Ethics committee (approval number: 18/2561) and in accordance with the Declaration of Helsinki, 72 university athletes, both male and female (18–30 years) were recruited.
Burapha university athletes in the Bangsaen Campus, Chonburi, Thailand were recruited in this study. Placards containing the study details were posted in the main areas of the Burapha University, such as the Faculty of Sport Science, the student dormitories, cafeteria, and library. Persons interested in participating in the study contacted a research assistant by phone and Line application. After making an appointment, they were screened through health questionnaires used to examine their general information, medical illness, history of exercise participation and supplementation intake, and mental health, in addition to a physical examination, which measured body mass, height, body mass index (BMI), blood pressure (BP), and heart rate (HR).
Prior to experiment commencement, each participant signed a written informed consent after completing study details. All participants were healthy of body and mind, were nonregular smokers or drinkers, free from medication and supplement intake, and of normal BMI. The participants were randomly recruited using a random number table into three groups (n=22/group): control group, RT group, and HIIT group as shown in Fig. 1.
The intervention design in the present study followed our previous protocol as referenced in Padkao and Prasertsri (2019), which was published in the Journal of Exercise Physiology online. (Padkao and Prasertsri, 2019). The control group was requested to maintain regular physical activity and dietary intake behaviors for 8 weeks. The RT group was instructed to perform a resistance exercise training program of the upper body (lateral pulldowns biceps curls, triceps extensions, seated dumbbell press, and lateral dumbbell raises) and lower body (leg press, lying leg curls, leg extensions, hip abductions, and adductions). In brief, the initial exercise was begun at 30% of 1RM in each muscle group, 15 repetitions/set/muscle group, then repeated at 70% of 1RM in each muscle group, 8 repetitions/set/muscle group, and finally performed at 30% of 1RM. Therefore, the total exercise session consisted of a couple of sets/muscle groups at low intensity (30% of 1RM) and a single set/muscle group at greater intensity (70% of 1RM). All subjects performed a 3-min warm-up and 3-min cool-down in the form of brisk marching. Exercise duration was 30 min/day, 3 days a week over 8 weeks. The HIIT group performed an exercise training program on a bicycle ergometer (Monark 828E ergomedic, Monark Exercise AB, Vansbro, Sweden) for 30 min/day, 3 days a week, for 8 weeks. Subjects began with a 3-min warm-up with free workload cycling. Then, a load was added to render 30% of maximal oxygen consumption (VO2max) for 1 min (low intensity). After that, it was increased to an intensity of 70% of VO2max (high intensity). Exercise intensity was interchanged between 30% and 70% VO2max every min for 24 min. During exercise, subjects were required to maintain a speed of 50–60 rpm/min. At exercise completion, they performed a 3-min cool-down with free workload. Exercise duration in this group was the same as in the RT group. Assessments of parameters at pre- and posttraining were performed within 3 days prior to and subsequent to the training at the same duration of the day i.e., in the morning.
One repetition maximum and VO2max measurementThe 1RM is defined as the maximal weight that can be lifted once with the correct lifting technique. It is considered the gold standard for evaluating muscle strength in nonlaboratory situations (Seo et al., 2012). Participants in the RT group were evaluated for 1RM in each muscle group as recently described (Padkao and Prasertsri, 2019).
VO2max was measured using a bicycle ergometer (Monark Ergomedic 828E, Monark Exercise AB, Sweden) according to the YMCA cycle test as previously defined (Padkao and Prasertsri, 2016).
Body composition and hemodynamics measurementBody composition and hemodynamics data were expressed as baseline characteristics for all participants. Body composition measurements were conducted in the standing position using a body composition analyzer (InBody 270, Ordamed Co., Ltd., Seoul, Korea) based on the principle of bioelectrical impedance analysis. Hemodynamic parameters were measured incorporating systolic BP (SBP), diastolic BP, mean arterial BP, and HR. HR pressure product (HRPP) was calculated by SBP multiplied by HR.
Plasma malondialdehyde measurementPlasma malondialdehyde (MDA) was determined by measuring thiobarbituric acid reactive substances as previously mentioned (Nakmareong et al., 2011). In brief, blood was collected in an ethylenediaminetetraacetic acid (EDTA) tube and centrifuged at 3,500 rpm, for 10 min, at 4°C. Plasma samples of 150 μL were combined with 10% trichloroacetic acid, 5 mM EDTA, 8% sodium dodecyl sulfate, and 0.5 μg/mL of butylated hydroxytoluene. This mixture was incubated for 10 min at room temperature with 500 μL of 0.6% 2-thiobarbituric acid added. The mixture was then boiled in a water bath for 30 min. After cooling to room temperature, the mixture was centrifuged at 10,000×g for 5 min. Supernatant absorbance was measured at 532 nm via a spectrophotometer. A standard curve was generated with appropriate concentrations of 1,1,3,3-tetraethoxypropane (0.3–10 μM).
Whole blood glutathione measurementWhole blood total glutathione (GSH) and Glutathione disulfide (GSSG) were measured in accordance with a previously described method (Sompamit et al., 2009). GSSG was determined using M2VP as a GSH scavenger. Briefly, a 100-μL whole blood sample was immediately reacted with 10 μL of 33 mM M2VP or distilled water and subsequently treated with 5% cold MPA to precipitate protein. Following centrifugation, the supernatant was used in the enzymatic coupling assay for GSH via a spectrophotometer (Ultrospec 6300 pro, Biochrom Ltd., Cambridge, UK). Lastly, redox ratio was calculated as GSH/GSSG.
Physical performance measurementPhysical performance, flexibility, muscle strength, and endurance were determined in each participant. For the sit and reach test, a box was utilized for the assessment of trunk and leg flexibility. The highest value from three maneuvers was recorded. Each participant was asked to perform the maneuver in the sitting position with the legs straight and knees flat against the floor. Individuals were subsequently instructed to lean forward and reach out toward the box slowly as far as possible and hold for 2 sec. A handheld dynamometer was employed to measure leg muscle strength. The greatest value from two maneuvers was recorded. Each participant was requested to perform the maneuver in the standing position with the head and trunk straight along with slight hip and knee flexion. Subjects were instructed to pull the handheld dynamometer as much as possible and hold for 2 sec. They were allowed to rest for one minute and then repeat the maneuver. Muscle endurance performance to the point of fatigue was conducted utilizing the barbell bench press method. The YMCA test was administered according to procedures as previously described. A 36-kg (80 lb) barbell was used for men, with a 16-kg (35 lb) barbell used for women. The bar was either raised or lowered with each beat to achieve an overall lifting pace of 30 reps/min via metronome. Subjects were instructed to maintain a controlled pace throughout the test. The test was terminated when they could no longer lift the bar or were unable to maintain cadence (Kim et al., 2002). The maximum amount of barbell bench press repetitions was recorded. Briefly, participants lie back on a flat bench and grip the bar at shoulder width apart. From the starting position, subjects were instructed to breathe in and begin moving the weight down slowly until the bar touched the middle of the chest. They then pushed the bar upwards with the arms fully extended while breathing out. Subjects repeated the movement as many times as possible according to the pace of the metronome.
Statistical analysisAll data are presented as means±standard deviation. Normal distribution of data was applied according to the Shapiro–Wilk test. Differences among various groups were compared using one-way analysis of variance followed by Bonferroni post hoc test to determine the variances between groups. Paired t-test was applied to determine the differences before and after within groups. A P-value of <0.05 was considered statistically significant.
RESULTSGeneral profile of participantsThe mean age of participants was 21.8±1.0, 20.4±0.8, and 20.3±0.6 years in control, RT, and HIIT groups, respectively. The body weight in HIIT group was significant difference compared with RT group (P=0.015). This might partly be due to the difference in male to female ratio that was higher in HIIT group (Table 1). Amount of habitual exercise performed by the control, RT, and HIIT groups were 355.5±285.1 min/wk, 309.0±237.0 min/wk, and 277.2±218.4 min/wk, respectively. There were no statistically significant differences between groups. Moreover, there were no significant differences in baseline height, BMI, fat mass, lean mass, and hemodynamics parameters in all experimental groups (Table 1).
Effect of exercise training on oxidative stress markersOxidative stress is defined as an excessive of oxidants over antioxidants within a body system. The direct consequence of this condition is a shift in the redox state of biological compartments including DNA, mitochondria, lipid, and protein toward oxidizing and cause tissue damage.
A common approach to determine oxidative stress in biological systems involves assessment of the decrease or increase in a biomarker molecule that responds to oxidative stress. Evaluation of tissue antioxidant or lipid tissue damage, which MDA is a key by-product, is commonly attribute. To protect against oxidative damage, increases in antioxidant production and activity play a chief role in scavenging and reducing ROS. Thus, decline in the tissue antioxidant level is considered as to be oxidative stress. Besides, major tissue antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase (GPX) are existing and inducible in skeletal muscles. Among these antioxidant enzymes, GPX is indicated to increase in skeletal muscle fibers that are actively recruited during regular exercise training (Powers and Jackson, 2008).
Plasma MDA, a biomarker of lipid peroxidation is one criterion which has been identified as concerning oxidative stress status. At the outset period, plasma MDA exhibited no significant difference amid all experimental groups. Interestingly, after 8 weeks of RT, a significant reduction of plasma MDA compared with baseline (P=0.016) (Fig. 2) was observed.
GPX is an intracellular antioxidant enzyme. A greater level of blood glutathione indicates increased antioxidant levels. All GPXs are enzymes that catalyze the reduction of H2O2 to water by using reduced GSH as the electron donor. When GSH is the electron donor, it donates a pair of hydrogen ions, and GSH is oxidized to GSSG. High ROS production results in a decrease in the GSH/GSSG ratio, indicating lower levels of reduced GSH in favor of increased oxidized GSH. Surprisingly, the results of this study demonstrated that GSH significantly increased postexperimental period in all experimental groups (P<0.05) (Fig. 3A). After 8 weeks of both resistance and HIIT, GSH/GSSG redox ratios were markedly increased compared with baseline (P<0.05) (Fig. 3B). Thus, this data suggests that both types of exercise intervention improved antioxidant status in athletes. However, GSH and GSH/GSSG ratio were not significantly different between groups over time.
DISCUSSIONThe main findings of the present study are that both long-term RT and HIIT improved blood glutathione levels and GSH/GSSG redox ratio in all athletes. Unexpectedly, our results demonstrated that only RT training significantly decreased plasma MDA. The effect of HIIT on plasma MDA showed no significant changed posttraining. Another finding of the present investigation was that an 8-week RT and HIIT program resulted in similar elicitation of physical performance including leg strength, back and leg flexibility, and upper body endurance in the post-compared with pretraining exercise.
ROS occur under both pathological and physiological conditions, thus causing direct or indirect tissue damage. In fact, oxygen intake is significantly raised during exercise due to increased muscular work. It has been reported that intense exercise may induce oxidative damage and that it is the major source of producing free radicals which damage muscle mitochondria (Finaud et al., 2006). Although free radicals are well under control, depletion of antioxidant defense might be a cause of muscle tissue damaged, and in turn, induce muscle fatigue and inhibit performance. Glutathione is the major antioxidant compound found in the intracellular compartment. It is present in both reduced GSH and oxidized GSSG forms acting in concert with other redox-active compounds such as NAD(P)H to regulate cellular redox status and directly react with ROS (Lushchak, 2012). This study revealed that after 8 weeks of training, GSH and GSH/GSSG were increased in both RT and HIIT groups. Indeed, glutathione elevation may be explained by increasing ROS during regular exercise, thus resulting in activating the adaptation of muscle oxidative capacity (Jackson, 2009) as well as promoting antioxidant capacity. Hence, this data suggests that both exercise types and exercise components (frequency, intensity, and duration) were optimal in promoting antioxidant defendants as well as improving physical performance in athletes.
These results are in accordance with previous findings whereby a single session of HIIT in healthy subjects comprising of 30-sec bouts of cycling separated by 4 min of recovery, at a total duration of 16 min induced increases in GPX, catalase, and total antioxidant activity in the post-compared with pretraining exercise test (Bogdanis et al., 2013). Moreover, long-term treadmill HIIT showed lower levels of serum, skeletal muscle MDA, and 8-hydroxydeoxyguanosine in the aged rat model (Li et al., 2018). In contrast, our study reported no significant changes in plasma MDA following a bicycle ergometer HIIT program incorporating 12 min of high intensity and 12 min of low intensity, 3 days a week, for 8 weeks. It would seem reasonable to assume that the volume of this program was higher compared to others (Bogdanis et al., 2013; Li et al., 2018). Thus, this may lead to increased ROS production during exercise training and result in increased oxidative stress. Therefore, this HIIT program might be not ideal to attenuate oxidative stress markers in athletes.
Although oxidative alteration in RT has been reported, the variety of volume and intensity of this training is quite different. For example, a healthy subject who performed 3 sets of 10 repetitions at 70% of 1 RM did not record alterations in oxidative stress markers and antioxidants. Other authors have demonstrated that high-intensity strength exercise increases the level of oxidative markers as well as decreasing antioxidant production in well-trained power-lifting athletes (Jurgenson et al., 2019). Notwithstanding, moderate to high RT has been reported to improve oxidative stress status in several participants (Azizbeigi et al., 2013; Azizbeigi et al., 2015; Parise et al., 2005; Ryrso et al., 2018). Our study established that the group of athletes who performed resistance exercise with a couple of sets of low intensity (30% of 1RM, 15 repetitions/set/muscle group) and a single set of high intensity (70% of 1RM, 8 repetitions/set/muscle group) exhibited significantly decreased plasma MDA as well as increased glutathione levels post 8-week training periods.
In recent years, the importance of RT has been emphasized in general health guidelines. RT is composed of both concentric (when the activated muscle shortens) and eccentric (when the activated muscle lengthens). Exercise-induced muscle damage occurs postexercise, particularly if this exercise involves a great number of eccentric contractions (Castrogiovanni and Imbesi, 2012). Direct signs of muscle damage include an increase in inflammatory response, both in muscle tissue and blood, a decrease in force production, an elevation of muscle proteins in the blood, and muscle soreness. Therefore, a strenuous repeated bout of resistance contraction may place the muscle under stressed conditions and promote tissue damage. Moreover, free radicals may be released from muscle cells. As previously stated, this data showed a decrease in plasma MDA and an increase in glutathione posttraining protocol. This adaptation occurs so that the muscle is more resistant to subsequent damage induced by exercise. Interestingly, evidence of this RT protocol may be advantageous amid improving physical performance and antioxidative adaptation in athletes.
Regarding the effects of RT and HIIT on physical performance in university sports athletes, we discovered that both RT and HIIT improved leg strength, back and leg flexibility, and upper body endurance in the post-compared with pretraining exercise. These findings suggest that either a couple of sets of low resistance (30% of 1RM) and a single of set of high resistance (70% of 1RM) or bicycle HIIT protocol can be employed to gain similar physical performance outcomes.
HIIT has been shown to improve aerobic fitness as similar as traditional endurance exercise, especially generating comparable improvement in VO2max. Moreover, some studies have reported that HIIT induced faster and more significant adaptations in VO2max when compared to traditional endurance or moderate-intensity continuous training (Arboleda-Serna et al., 2019; Helgerud et al., 2007; Menz et al., 2019; Wisloff et al., 2007; Ziemann et al., 2011). As described above, HIIT was anticipated to enhance endurance fitness in this study.
Certain limitations concerning this study need to be considered. Firstly, the present study lacked a true control group, i.e., subjects would have already been participating in resistance, aerobic, and, or field training routines themselves. Secondly, we did not control dietary intake during the study, however, we advised subjects not to change their usual nutritional habits throughout the experimental period. Finally, as a present result, we cannot guarantee that the RT program applied in this study is not superior to the HIIT program. Regarding the total time of high-intensity training in the HIIT group, it was greater than in the RT group. What’s more, our previous results revealed that HIIT was superior to RT in regards improvements in body composition and cardiovascular responses in university sports athletes (Padkao and Prasertsri, 2019).
In conclusion, the present study revealed that RT and HIIT programs improve antioxidant glutathione as well as physical performance in university sports athletes. Notwithstanding, oxidative markers were only improved following the RT program. Hence, this study suggests that a RT program is superior to HIIT in terms of improving oxidative stress among sports athletes. Nevertheless, future studies with differences in program design variable such as volume and intensity ought to be considered to confirm the effects of RT and HIIT on oxidative stress markers.
ACKNOWLEDGMENTSThis research was supported by grants from the Faculty of Allied Health Sciences, and the Exercise and Nutrition Innovation and Sciences Research Unit, Burapha University, Thailand.
REFERENCESArboleda-Serna VH, Feito Y, Patino-Villada FA, Vargas-Romero AV, Arango-Velez EF. Effects of high-intensity interval training compared to moderate-intensity continuous training on maximal oxygen consumption and blood pressure in healthy men: a randomized controlled trial. Biomedica. 2019;39:524–536.
Azizbeigi K, Azarbayjani MA, Atashak S, Stannard SR. Effect of moderate and high resistance training intensity on indices of inflammatory and oxidative stress. Res Sports Med. 2015;23:73–87.
Azizbeigi K, Azarbayjani MA, Peeri M, Agha-alinejad H, Stannard S. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int J Sport Nutr Exerc Metab. 2013;23:230–238.
Billat LV. Interval training for performance: a scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: aerobic interval training. Sports Med. 2001;31:13–31.
Bloomer RJ, Goldfarb AH, McKenzie MJ. Oxidative stress response to aerobic exercise: comparison of antioxidant supplements. Med Sci Sports Exerc. 2006;38:1098–1105.
Bogdanis GC, Stavrinou P, Fatouros IG, Philippou A, Chatzinikolaou A, Draganidis D, Ermidis G, Maridaki M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol. 2013;61:171–177.
Buchheit M, Mendez-Villanueva A, Quod M, Quesnel T, Ahmaidi S. Improving acceleration and repeated sprint ability in well-trained adolescent handball players: speed versus sprint interval training. Int J Sports Physiol Perform. 2010;5:152–164.
Castrogiovanni P, Imbesi R. Oxidative stress and skeletal muscle in exercise. Ital J Anat Embryol. 2012;117:107–117.
Finaud J, Lac G, Filaire E. Oxidative stress : relationship with exercise and training. Sports Med. 2006;36:327–358.
Gist NH, Freese EC, Ryan TE, Cureton KJ. Effects of low-volume, high-intensity whole-body calisthenics on army ROTC cadets. Mil Med. 2015;180:492–498.
Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665–671.
Higgins MR, Izadi A, Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Public Health. 2020;17:8452
Jackson MJ. Strategies for reducing oxidative damage in ageing skeletal muscle. Adv Drug Deliv Rev. 2009;61:1363–1368.
Jurgenson J, Serg M, Kampus P, Kals J, Zagura M, Viru M, Zilmer K, Zilmer M, Eha J, Unt E. Oxidative stress parameters and its associations with arterial stiffness in competitive powerlifting athletes after 12-week supervised strength training. J Strength Cond Res. 2019;33:1816–1822.
Kim PS, Mayhew JL, Peterson DF. A modified YMCA bench press test as a predictor of 1 repetition maximum bench press strength. J Strength Cond Res. 2002;16:440–445.
Koozehchian MS, Daneshfar A, Fallah E, Agha-Alinejad H, Samadi M, Kaviani M, Kaveh BM, Jung YP, Sablouei MH, Moradi N, Earnest CP, Chandler TJ, Kreider RB. Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males. J Exerc Nutrition Biochem. 2018;22:7–19.
Li FH, Sun L, Zhu M, Li T, Gao HE, Wu DS, Zhu L, Duan R, Liu TC. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Exp Gerontol. 2018;113:150–162.
Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:736837
Mastaloudis A, Yu TW, O’Donnell RP, Frei B, Dashwood RH, Traber MG. Endurance exercise results in DNA damage as detected by the comet assay. Free Radic Biol Med. 2004;36:966–975.
Menz V, Marterer N, Amin SB, Faulhaber M, Hansen AB, Lawley JS. Functional Vs. running low-volume high-intensity interval training: effects on VO2max and muscular endurance. J Sports Sci Med. 2019;18:497–504.
Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, Sompamit K, Phisalaphong C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn-Schmied Arch Pharmacol. 2011;383:519–529.
Padkao T, Prasertsri P. Effectiveness of an upper and lower limb resistance training program on body composition, nerve conduction velocity, and cardiac autonomic nervous activity in university athletes. J Exerc Physiol Online. 2019;22:78–98.
Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med. 2005;39:289–295.
Park SY, Kwak YS. Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes. J Exerc Rehabil. 2016;12:113–117.
Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276.
Ryrso CK, Thaning P, Siebenmann C, Lundby C, Lange P, Pedersen BK, Hellsten Y, Iepsen UW. Effect of endurance versus resistance training on local muscle and systemic inflammation and oxidative stress in COPD. Scand J Med Sci Sports. 2018;28:2339–2348.
Seo DI, Kim E, Fahs CA, Rossow L, Young K, Ferguson SL, Thiebaud R, Sherk VD, Loenneke JP, Kim D, Lee MK, Choi KH, Bemben DA, Bemben MG, So WY. Reliability of the one-repetition maximum test based on muscle group and gender. J Sports Sci Med. 2012;11:221–225.
Sompamit K, Kukongviriyapan U, Nakmareong S, Pannangpetch P, Kukongviriyapan V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur J Pharmacol. 2009;616:192–199.
Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094.
Fig. 2Plasma malondialdehyde in all experimental groups. Values are presented as mean±standard deviation (n=20/group). Con, control; HII-RT, high-intensity interval-resistance training; HII-CT, high-intensity interval-cycling training. *P<0.05 vs. baseline. 
Fig. 3Effect of exercise training on blood glutathione levels (GSH) (A) and the redox ratio of glutathione disulfide (GSH/GSSG) (B) of subjects in all experimental groups. Values are presented as mean±standard deviation (n=20/group). Con, control; HII-RT, high-intensity interval-resistance training; HII-CT, high-intensity interval-cycling training. *P<0.05 before vs. after. 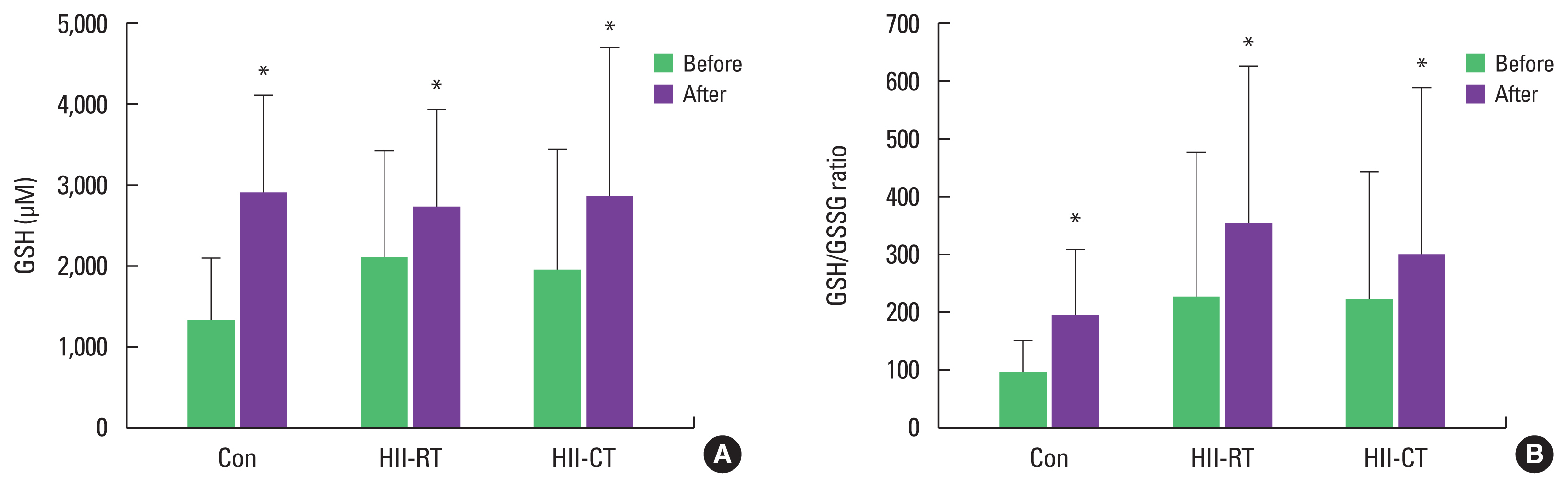
Fig. 4Effect of exercise training on flexibility of subjects in all experimental groups. Values are presented as mean±standard deviation (n=20/group). (A) Control group. (B) High-intensity interval-resistance training (HII-RT) group. (C) High-intensity interval-cycling training (HII-CT) group. *P<0.05 before vs. after. 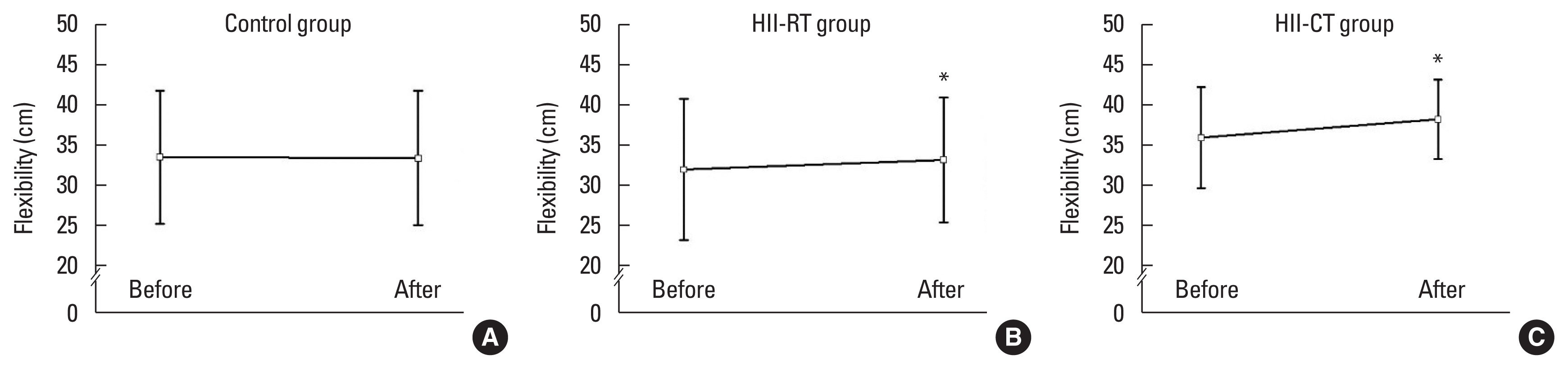
Fig. 5Effect of exercise training on leg strength of subjects in all experimental groups. Values are presented as mean±standard deviation (n=20/group). (A) Control group. (B) High-intensity interval-resistance training (HII-RT) group. (C) High-intensity interval-cycling training (HII-CT) group. *P<0.05 before vs. after. 
Fig. 6Effect of exercise training on barbell bench press test of subjects in all experimental groups. Values are presented as mean±standard deviation (n=20/group). (A) Control group. (B) High-intensity interval-resistance training (HII-RT) group. (C) High-intensity interval-cycling training (HII-CT) group. *P<0.05 before vs. after. 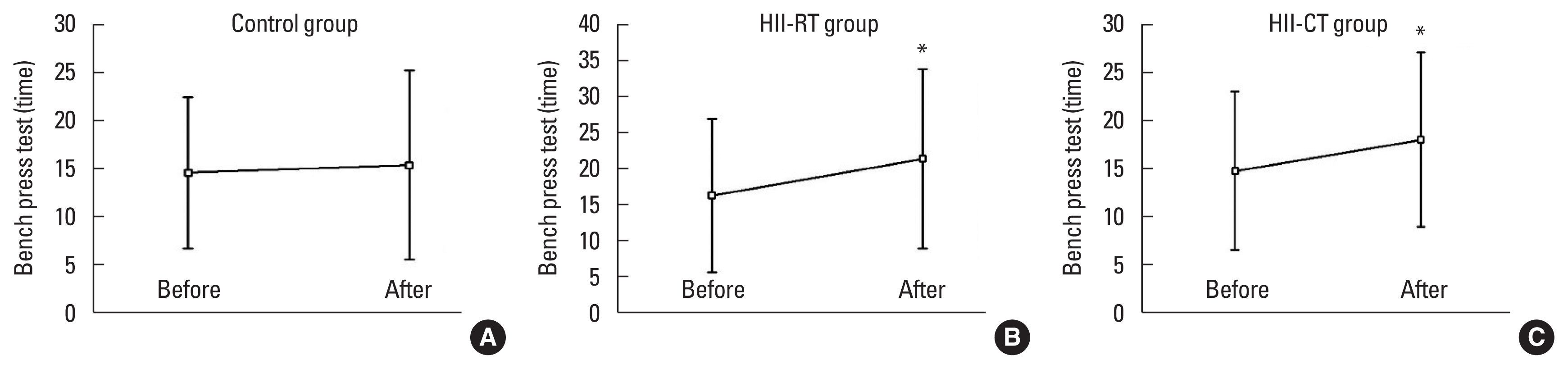
Table 1General profile of all the subjects
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||