AbstractThis study aimed to investigate the adequacy of the visual-motor inte-gration (VMI) scale for Korean elderly (VMIS-KE) compared to tradition-al measures, mini-mental state examination of Korean version (MMSE-KC) and Beery VMI for cognitive decline in diabetic older adults. For this explanatory research, data were collected from September 1 to Sep-tember 15, 2013, from 34 diabetic older adults and 31 nondiabetic older adults in Daegu and Gyeongsan of Korea. Mean differences between the two groups were analyzed with SPSS 18.0. The diabetic older adults showed significantly lower scores in the VMIS-KE (t=4.128, P<0.001) and MMSE-KC (t=2.231, P=0.029) compared with the nondiabetic older adults. In all subcategories of VMI-KE, figure cognition (t=5.342, P<0.001), memory (t=3.011, P=0.004) and spatial cognition (t=2.639, P=0.011), there were significant differences whereas no significant difference in the VMI-6th revision (t=0.994, P=0.325). VMIS-KE could be a sensitive indicator to assess cognitive change in older adults with diabetes and health care providers should periodically evaluate vulnerable groups such as them with it.
INTRODUCTIONAs age increases, cognitive function declines, and in particular, older adults after the age of 70 show a significant cognitive decline (Park et al., 2021). Aging is one of the risk factors for dementia (Grande et al., 2020). Dementia is caused by biological mechanisms such as neurodegeneration, brain resilience, vascular damage, inflammation, and oxidative stress. Among them, oxidative damage to the brain is associated with diabetes (Grande et al., 2020). Chronic hyperglycemia in diabetes leads to increases advanced glycation end products that cause oxidative damage and this glucotoxicity results in neuronal damage and dopamine dysfunction (Pignalosa et al., 2021). Furthermore, diabetes could contribute to asymmetric atrophy of the hippocampus (Milne et al., 2017). In a longitudinal cohort study, diabetes was significantly related to subsequent dementia (Barbiellini Amidei et al., 2021). In the diabetics, physical activity is closely associated with cognitive decline (Midorikawa et al., 2021). Physical activity particularly influences to episodic memory function and can predict cognitive decline after 2 years (Bai et al., 2021). Accordingly, health care providers should assess cognitive impairment of the diabetics with screening tools including physical activity at an early stage (Bai et al., 2021).
Mini-mental state examination (MMSE) developed by Folstein et al. (1975) is a most widely used cognitive function test because it is quick and easy to use. It is useful to assess the severity and change of cognitive function (Folstein et al., 1975). However, it has limitation to detection of early dementia with mild cognitive impairment (Arevalo-Rodriguez et al., 2015; Pinto et al., 2019), and should interpret with considering education level of participants (Lee et al., 2002). Cognitive decline in diabetes has a greater effect on attention, processing speed and executive functions than on memory and there are many diabetic patients undiagnosed cognitive impairment in diabetic clinics (Bai et al., 2021; Schimming et al., 2017). More sensitive measure and periodic evaluation for cognitive decline of diabetic older adults are required (Bai et al., 2021).
Sensory processing tasking could be a more sensitive measure than traditional cognitive function tests in diabetic older adults (Humes, 2016) because diabetes deteriorated visual-somatosensory integration and motor function (Mahoney et al., 2021). Visual-somatosensory integration in older adults is associated with cognitive impairment (Mahoney and Verghese, 2020). Visual-motor integration (VMI) is a vision-related cognitive function that is a degree of coordination between visual perception and finger-hand movements (Bolk et al., 2018). The Beery VMI is a valuable measure from children (Bolk et al., 2018) to older adults and can be an early indicator of cognitive decline (Yun et al., 2013). However, it strongly focused on childhood academic achievement (Osorio-Valencia et al., 2018). Especially older adults with blurred vision had difficulty in responding accurately and adjusting the print size of the materials had to be considered to respond comfortably (Findlay et al., 2020).
The VMI scale for Korean elderly (VMIS-KE) is the first measure to assess cognitive decline through VMI for Korean older adults, its validity and reliability were demonstrated, and differences in cognitive function by aging could be detected regardless of educational level (Park et al., 2021). Therefore, this study aims to verify the effectiveness of VMIS-KE compared to traditional tools, MMSE and VMI-6th revision (VMI-6R), as an early screening tool for cognitive decline in diabetic older adults.
MATERIALS AND METHODSDesignThis study was an explanatory survey research to determine the adequacy of VMIS-KE by comparing the traditional measurements, MMSE of Korean version (MMSE-KC) and VMI-6R, to detect cognitive decline for diabetic and nondiabetic older adults. To achieve the purpose of this study, MMSE-KC, VMI-6R, and VMIS-KE scores of diabetic and nondiabetic older adults were investigated and compared at 1 timepoint.
Subjects and data collectionTo determine the difference in independent variables between diabetic and nondiabetic older adults, the required number of samples in the condition of two sample t-test, medium effect size 0.50, significance level 0.05, and power 0.90 in the G power 3.1.9.7 program was 30 per group, total 60 participants. The criteria for selecting subjects for this study were (a) older adults over 65, (b) who understood the purpose of this study and consented to participate in the study, (c) who can communicate without any audiovisual disabilities, and (d) who were not diagnosed with neuropsychiatric disorders including dementia. For this study, data were collected from September 1 to September 15, 2013, from 34 diabetic older adults visiting Kyungpook National University hospital for ambulatory care and from 31 nondiabetic older adults visiting welfare facilities for the older adults in Daegu and Gyeongsan of Korea. All participants completed the face-to-face interview and questionnaires according to the direction of the research assistants trained for this study. It took about 20 min to complete this survey.
MMSE-KCCognitive function was measured by MMSE-KC (Lee et al., 2002). It includes eight categories, time orientation, place orientation, memory registration, attention/calculation, memory recall, language function, visuo-spatial construction, and understanding/judgment. The range of scores is from 0 to 30 and higher means better cognitive function. The Cronbach alpha coefficient was 0.92 in the study by Lee et al. (2002) and 0.766 in this study.
VMI-6R
Beery and Beery (2010) developed VMI-6R to assess VMI. It consists of a total of 24 questions to assess the level of integrated ability between vision and exercise. Participants are asked to draw along geometric shapes presented in the upper column with a pencil. The score is 0 point if the shape is incorrect based on the grading criteria, and 1 point if it is correct. The total score ranges between 0 and 30 points, and the higher the score, the higher the VMI. The Cronbach alpha coefficient was 0.94 and the test-retest reliability was 0.88 for subjects aged 60 to 69 years in the study by Beery and Beery (2010), and the Cronbach alpha coefficient was 0.806 in this study.
VMIS-KEVMIS-KE is the VMI scale for the Korean elderly developed by Park et al. (2021) to detect cognitive decline in the early stage of Korean older adults. It consists of 12 items classified into three categories, figure cognition, memory, and spatial cognition. Participants are asked to recognize the shape of a given figure and draw along or complete it in five figure cognition items, to recall and draw the figure remembered or to respond appropriately with remembering the condition in four memory items, and to draw lines or figures in a designated space in three spatial cognition items. Each item scores from 0 to 3 points based on the grading criteria. The total scores range from 0 to 36 points and the higher means better VMI. The Cronbach alpha coefficient was 0.867 in the study by Park et al. (2021) and 0.846 in this study.
Ethical considerationsResearchers explained the purpose, procedures, risks, and benefits of this study to the subjects. Every subject voluntarily participated in this study with informed consent. Collected data were used for purpose of the study and it was informed that participation could be withdrawn at any time. Researchers provided souvenirs to all participants as an appreciation for their participation.
Data analysisData were analyzed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics for general characteristics of participants were conducted with mean, standard deviation, frequency, and percentage. Homogeneity tests for general characteristics between groups were conducted with chi-square test, Fisher exact test and two sample t-test. The normality distribution of each group was verified with the Kolmogorov–Smirnov test. Difference tests for means of the MMSE-KC, VMI-6R, and VMIS-KE between diabetic and nondiabetic older adults were conducted with two sample t-test. The statistical significance level in this study was set at P<0.05.
RESULTSGeneral characteristics and homogeneity tests between nondiabetic and diabetic older adultsThe study sample comprised 65 participants, 31 in the nondiabetes and 34 in the diabetes group. All variables showed approximately normal distributions, including age, gender, educational periods, families living together, subjective health status, smoking, drinking, exercise, depression, activities of daily living, and instrumental activities of daily living. There were no significant differences with respect to any variable between the nondiabetes and diabetes groups except subjective health status (χ2=9.71, P=0.013). Table 1 summarizes the general characteristics of the participants.
The mean difference of the MMSE-KC between nondiabetic and diabetic older adultsThe mean scores of the MMSE-KC were described in Fig. 1. The mean scores in the nondiabetic and diabetic older adults were respectively 26.19±2.40 and 24.58±3.28 and that of the diabetic group was significantly lower than that of the nondiabetic group (t=2.231, P=0.029).
The mean difference of the VMI-6R between nondiabetic and diabetic older adultsThe mean scores of the VMI-6R were described in Fig. 2. The mean scores in the nondiabetic and diabetic older adults were respectively 29.39±11.01 and 26.94±9.41 and that of the diabetic group was lower than that of the nondiabetic group but the difference was not statistically significant (t=0.994, P=0.325).
The mean difference of the VMIS-KE between the nondiabetic and diabetic older adultsThe mean scores of the VMI-KE were described in Fig. 3. The total mean scores in the nondiabetic and diabetic older adults were respectively 21.90±5.61 and 15.62±6.57 and that of the diabetic group was significantly lower than that of the nondiabetic group (t=4.128, P<0.001).
In three subcategories of the VMI-KE, the mean scores in the nondiabetic group and the diabetic group were respectively as follows: figure cognition, 7.39±1.84 and 5.15±1.54; memory, 7.58±3.61 and 4.79±3.83; spatial cognition, 6.94±1.61 and 5.68±2.21. The diabetic older adults had significantly lower mean scores in figure cognition (t=5.342, P<0.001), memory (t=3.011, P=0.004), and spatial cognition (t=2.639, P=0.011), all subcategories of the VIMS-KE, than the nondiabetics.
DISCUSSIONThis study was conducted to verify the effectiveness of VMIS-KE as an early screening tool for cognitive decline in diabetic older adults. In this study, the cognitive function and VMI of diabetic and nondiabetic older adults were measured with VMIS-KE and the traditional tools, MMSE-KC and VMI-6R.
As a result of verifying the difference in cognitive function between the diabetic and nondiabetic older adults using MMSE-KC, the average of the diabetics was 1.61 lower than that of the nondiabetics, and the difference was significant. Diabetes may contribute to cognitive decline due to asymmetric atrophy of the hippocampus (Milne et al., 2017). Like this study, in the results of a previous study that measured the cognitive function of the older adults at a diabetes clinic by MMSE, the cognitive function of the diabetics was significantly lower than that of the nondiabetics (Schimming et al., 2017). Although diabetes is associated with cognitive decline (Callisaya et al., 2019; Danna et al., 2016), the diagnosis of cognitive decline in diabetes has not been made properly (Bai et al., 2021; Schimming et al., 2017). Cognitive decline in diabetes affects attention, processing speed, and executive functions rather than immediate and delayed recall functions, unlike amnestic type mild cognitive impairment. (Schimming et al., 2017). Therefore, a screening tool to more sensitively and early assess the cognitive decline of the diabetic older adults and periodic evaluation of their cognitive function of them are required (Bai et al., 2021).
In the VMI-6R, there was no significant difference between the diabetic and nondiabetic older adults even though the average of the diabetics was 2.45 lower than that of the nondiabetics. VMI has been mainly used as a tool for diagnosing cognitive achievement for children’s future academic success (Farhi et al., 2021; Spiridigliozzi et al., 2017). Although Beery’s VMI has been used to measure VMI in adults, it could be difficult for older adults with a blurred vision to respond accurately. In order to increase the accuracy, the print size of the materials should be adjusted so that older adults with presbyopia could respond comfortably (Findlay et al., 2020). Diabetes causes axon damage to the corticospinal tract and neurons (Muramatsu, 2020) and adversely influences visual-somatosensory integration, which mediates to decrease in motor function in diabetic older adults (Mahoney et al., 2021). Contrary to the results of this study, the VMI of diabetic older adults was significantly lower along with cognitive function (Yun et al., 2013). In a retrospective study (Humes, 2016), sensory processing tasking found differences more sensitively than cognitive function tests comparing the diabetic and nondiabetic older adults. Therefore, with a comfortable reading print size, verification of the difference in VMI between diabetic and nondiabetic older adults needs to be reconfirmed through future studies.
Finally, in the VMI-KE, the average of the diabetic older adults was 6.28 lower than that of the nondiabetics and there was a significant difference between the two groups. In all three subcategories of the VMI-KE, the diabetic older adults had significantly lower figure cognition, memory, and spatial cognition scores compared to the nondiabetic older adults. VMIS-KE was originally developed for early assessing cognitive decline of older adults and demonstrated significant differences by age groups and correlation with cognitive function (Park et al., 2021). Diabetes affects the central nervous system including the cerebral cortex, cerebellum, and basal ganglia, and decreases somatosensory and motor function (Ferris et al., 2020). Especially, visual-somatosensory integration and motor function could deteriorate in diabetic older adults (Mahoney et al., 2021). Sensory processing tasking could detect differences more sensitively than cognitive function tests between diabetic and nondiabetic older adults (Humes, 2016). MMSE, which is traditionally widely used to measure cognitive function, has limitations in finding such differences. Therefore, a vulnerable group such as older adults with diabetes should be periodically assessed with a more appropriate tool such as VMIS-KE that measures cognitive function with VMI.
This study demonstrated that older adults with diabetes had a poor cognitive function and VMI compared to older adults without diabetes and VMIS-KE was an effective method to detect these changes in the early stage. Health care providers should periodically evaluate cognitive decline in older adults and, particularly if they have diabetes, assess them with more sensitive sensorimotor process tasks, such as VMI, along with a general cognitive function test.
However, since this study was conducted on a small number of older adults in one area, care should be taken in generalizing the results of this study to the entire elderly population. We suggest a repeated study that includes more diverse and large-size subjects as a sample and a longitudinal study to observe changes in cognitive function including VMI in diabetic older adults.
ACKNOWLEDGMENTSThis work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012-S1A5B6034110).
REFERENCESArevalo-Rodriguez I, Smailagic N, Roque i, Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. Mini-mental state examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;3:CD10783
Bai A, Tao L, Huang J, Tao J, Liu J. Effects of physical activity on cognitive function among patients with diabetes in China: a nationally longitudinal study. BMC Public Health. 2021;21:481
Barbiellini Amidei C, Fayosse A, Dumurgier J, Machado-Fragua MD, Tabak AG, Sloten T, Kivimaki M, Dugravot A, Sabia S, Singh-Manoux A. Association between age at diabetes onset and subsequent risk of dementia. JAMA. 2021;325:1640–1649.
Beery KE, Beery NA. The Beery-Buktenica developmental test of visual-motor integration with supplemental developmental tests of visual perception and motor coordination. 6th ed. Bloomington (MN): Psych Corp; 2010.
Bolk J, Padilla N, Forsman L, Broström L, Hellgren K, Åden U. Visual-motor integration and fine motor skills at 6½ years of age and associations with neonatal brain volumes in children born extremely preterm in Sweden: a population-based cohort study. BMJ Open. 2018;8:e020478
Callisaya ML, Beare R, Moran C, Phan T, Wang W, Srikanth VK. Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: a longitudinal study. Diabetologia. 2019;62:448–458.
Danna SM, Graham E, Burns RJ, Deschênes SS, Schmitz N. Association between depressive symptoms and cognitive function in persons with diabetes mellitus: a systematic review. PLoS One. 2016;11:e0160809
Farhi A, Gabis L, Frank S, Glasser S, Hirsh-Yechezkel G, Brinton L, Scoccia B, Ron-Ei R, Orvieto R, Lerner-Geva L. Cognitive achievements in school-age children born following assisted reproductive technology treatments: a prospective study. Early Hum Dev. 2021;155:105327
Ferris JK, Inglis JT, Madden KM, Boyd LA. Brain and body: a review of central nervous system contributions to movement impairments in diabetes. Diabetes. 2020;69:3–11.
Findlay R, Black J, van der Werf B, Chelimo C, Grant CC, Anstice N. The effect of induced blur on the Beery-Buktenica developmental test of visual-motor integration and its supplemental tests. PLoS One. 2020;15:e0237807
Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198.
Grande G, Qiu C, Fratiglioni L. Prevention of dementia in an ageing world: evidence and biological rationale. Ageing Res Rev. 2020;64:101045
Humes LE. A retrospective examination of the effect of diabetes on sensory processing in older adults. Am J Audiol. 2016;25:364–367.
Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, Lee KH, Kim SY, Woo JI. Development of the Korean version of the consortium to establish a registry for Alzheimer’s disease assessment packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57:47–53.
Mahoney JR, Verghese J. Does cognitive impairment influence visual-somatosensory integration and mobility in older adults? J Gerontol A Biol Sci Med Sci. 2020;75:581–588.
Mahoney JR, Verghese J, George C. The influence of diabetes on multisensory integration and mobility in aging. Brain Sci. 2021;11:285
Midorikawa M, Suzuki H, Suzuki Y, Yamauchi K, Sato H, Nemoto K, Sugano Y, Iwasaki H, Sekiya M, Yatoh S, Tahagi N, Hada Y, Arai T, Shimano H. Relationships between cognitive function and odor identification, balance capability, and muscle strength in middle-aged persons with and without type 2 diabetes. J Diabetes Res. 2021;2021:9961612
Milne NT, Bucks RS, Davis WA, Davis TME, Pierson R, Starkstein SE, Bruce DG. Hippocampal atrophy, asymmetry, and cognition in type 2 diabetes mellitus. Brain Behav. 2017;8:e00741
Osorio-Valencia E, Torres-Sánchez L, López-Carrillo L, Rothenberg SJ, Schnaas L. Early motor development and cognitive abilities among Mexican preschoolers. Child Neuropsychol. 2018;24:1015–1025.
Park MS, Park YK, Kim EH, Kim H. Development of visual-motor integration scale for the Korean old people. J Exerc Rehabil. 2021;17:279–286.
Pignalosa FC, Desiderio A, Mirra P, Nigro C, Perruolo G, Ulianich L, Formisano P, Beguinot F, Miele C, Napoli R, Fiory F. Diabetes and cognitive impairment: a role for glucotoxicity and dopaminergic dysfunction. Int J Mol Sci. 2021;22:12366
Pinto TC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa ML, Ximenes RC, Sougey EB. Is the Montreal cognitive assessment (MoCA) screening superior to the mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31:491–504.
Schimming C, Luo X, Zhang C, Sano M. Cognitive performance of older adults in a specialized diabetes clinic. J Diabetes. 2017;9:929–935.
Fig. 1The mean difference of MMSE-KC between nondiabetic and diabetic older adults. Data are expressed as mean±standard deviation. Non-DM, nondiabetic older adults; DM, diabetic older adults; MMSE-KC, mini-mental state examination of Korean version. *P<0.05 compared with the nondiabetic older adults. 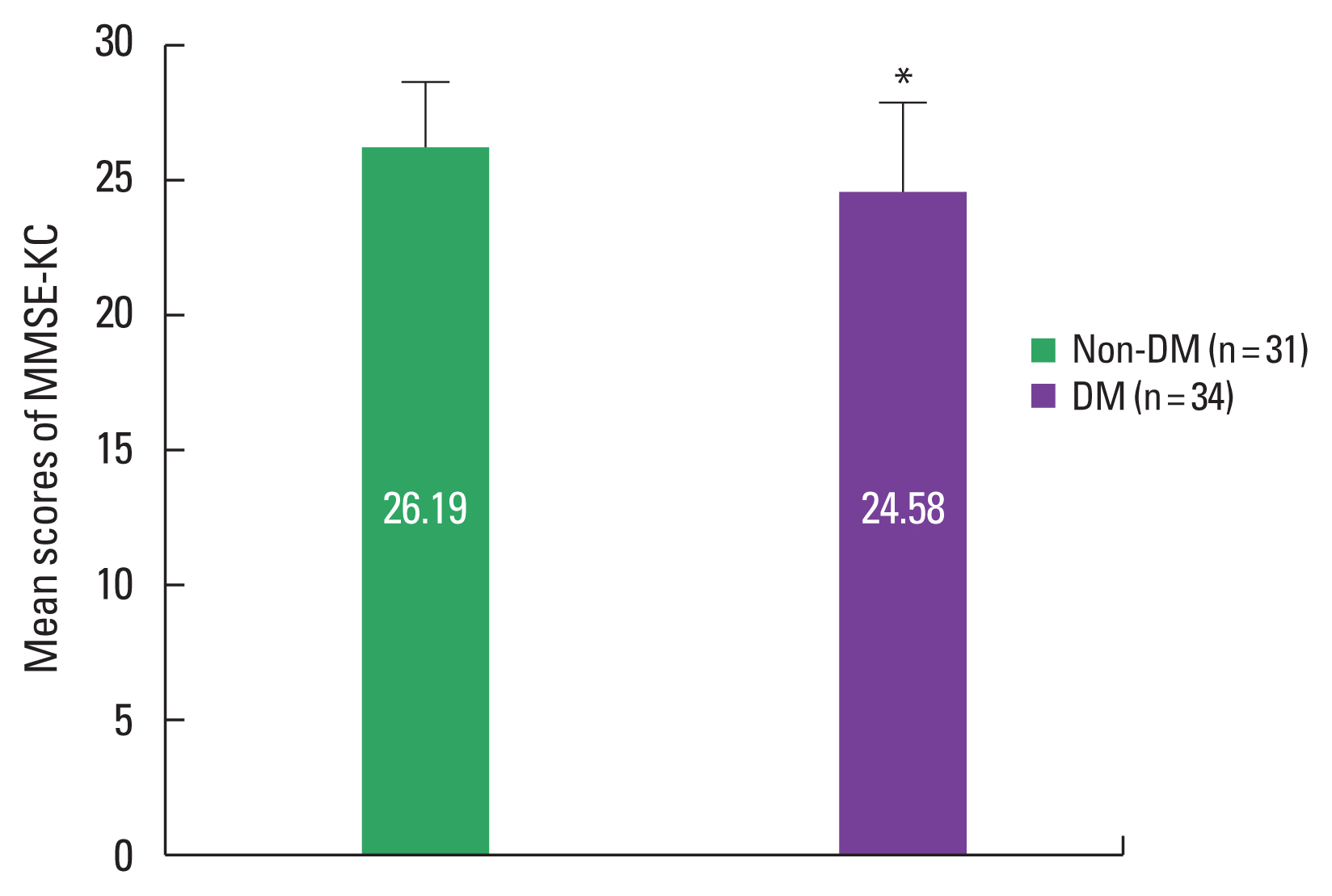
Fig. 2The mean difference of VMI-6R between nondiabetic and diabetic older adults. Data are expressed as mean±standard deviation. The difference was not statistically significant. Non-DM, nondiabetic older adults; DM, diabetic older adults; VMI-6R, visual-motor integration-6th revision. 
Fig. 3The mean difference of VMIS-KE between nondiabetic and diabetic older adults. 1 is the overall score of VMIS-KE, 2, 3, 4 are the subcategory scores of VMIS-KE (2, figure cognition; 3, memory; 4, spatial cognition). Data are expressed as mean±standard deviation. Non-DM, nondiabetic older adults; DM, diabetic older adults; VMIS-KE, visual-motor integration scale for Korean elderly. *P<0.05 compared with the nondiabetic older adults. 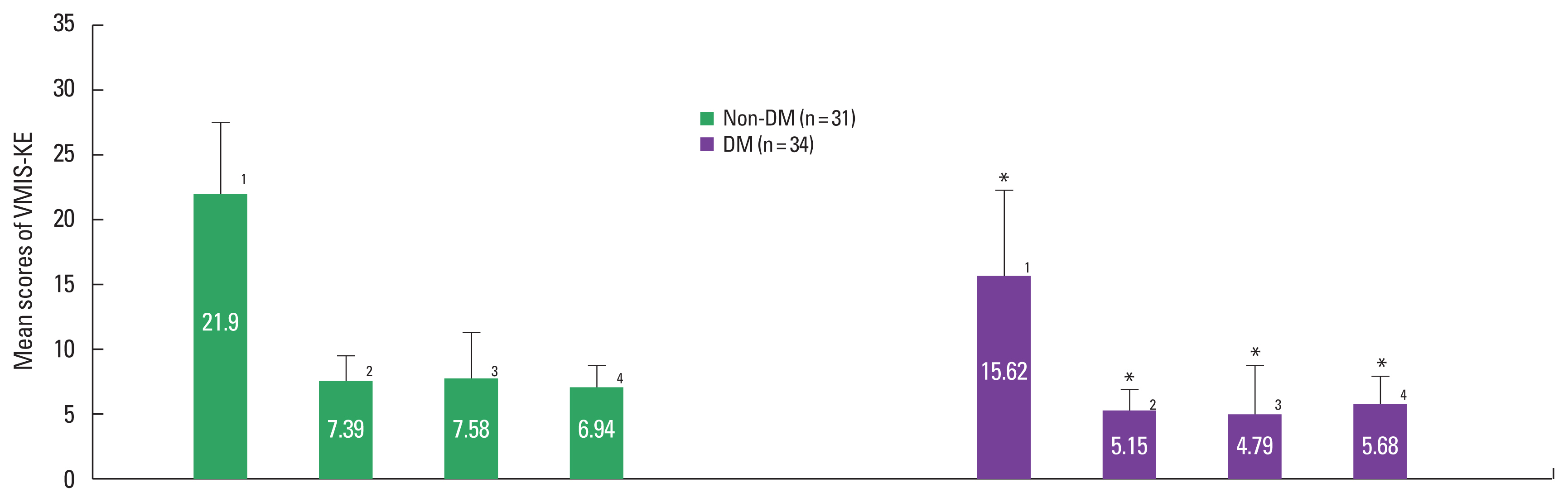
Table 1General characteristics and homogeneity tests between nondiabetic and diabetic older adults (N=65)
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||