INTRODUCTION
The sciatic nerve, which belongs to the peripheral nervous system capable of spontaneous regeneration, is damaged by femoral dislocation and fracture, or by muscle contusion and pressure in sports situations (Stafford et al., 2007). This sciatic nerve injury (SNI) causes a limitation in physical activity due to the degeneration of motor and sensory nerve axons and death of Schwann cells around the injury site (Richner et al., 2014).
Many previous studies on sciatic nerve regeneration have focused on axonal growth through the facilitation of Schwann cell proliferation and differentiation in the distal part of the injured area (Wang et al., 2015). Genetic overexpression of neurotrophic factors (Chen et al., 2010), activation of cell division cycle (Tanabe et al., 2003), stem cell transplantation (Dadon-Nachum et al., 2011) and regular physical activity (Bobinski et al., 2011) are suggested as therapeutic strategies for SNI. In the field of cell transplantation treatment, stem cell, neural cell, skin- and adipose-derived stem cell as well as bone marrow stromal cell (BMSC) have been mainly applied for central and peripheral nerve regeneration. Among them, autologous BMSC transplantation after SNI showed positive effects on proliferation of Schwann cell, nerve fiber elongation and pain-related protein expression in the dorsal root ganglion neurons without side effects (Joshi et al., 2021; Mimura et al., 2004). Also, regular low-intensity physical activity and exercise after SNI result in functional recovery and histological improvement by increasing the expression levels of regeneration-related proteins such as brain-derived neurotrophic factor, nerve growth factor, and glial cell line-derived neurotrophic factor in proliferating Schwann cells (Almeida et al., 2015; Gordon and English, 2016). Although these previous research findings have suggested that BMSC engraft or regular exercise might be a treatment method that promotes sciatic nerve regeneration, reliable studies on the synergistic effect of concurrent exercise and BMSC transplantation have not yet been published.
The sciatic nerve indirectly innervates all the skeletal muscles of the lower limbs, and patients with SNI experience limited physical activity due to pain and rapid muscle atrophy (Kobayashi et al., 2004; Malanotte et al., 2017). Muscle atrophy occurs when the rate of protein degradation exceeds protein synthesis, leading to decreased protein content, glycogen storage and oxygen availability in skeletal muscle (Schiaffino et al., 2021). In terms of biomechanical muscle metabolism, mammalian target of rapamycin (mTOR) signaling network is a major regulator of protein synthesis in skeletal muscle and myogenic regulatory factor (MRF) is basic helix-loop-helix transcription factors that prevent muscle atrophy composed of myoblast determination protein 1 (MyoD), myogenin, myogenic factor 4, 5, and 6.
Among the MRF transcription factors, MyoD and myogenin are muscle-specific proteins involved in muscle hyperplasia and hypertrophy (Hernández-Hernández et al., 2017). During muscle hypertrophy, MyoD is phosphorylated at an early stage of myogenic differentiation, and myogenin is subsequently activated at late stage. Legerlotz and Smith (2008) confirmed that MyoD was downregulated in denervated and disused muscle, but exercised muscle elevated the induction level of MyoD in vivo and in vitro experiments. In addition, Machida et al. (2012) reported that myogenesis-related protein including MyoD and myogenin, and mTOR downstream proteins were decreased at the beginning of denervation-induced skeletal muscle atrophy, but two signaling molecules tended to be activated 2 weeks later.
With these results presented by previous studies, both signaling pathways are key indicator for verifying muscle atrophy after SNI, and it is thought that exercise can improve muscle metabolism via activating these signaling downstream molecules. However, there is still a lack of studies observing the effects of combined low-volume exercise and BMSC transplantation on biochemical changes from the injured sciatic nerve to the soleus muscle after SNI, and on time-dependent changes in muscle hypertrophy-related proteins for a long time after injury. Therefore, the purpose of this study was to investigate the time-dependent alteration in whether concurrent low-intensity aerobic exercise and BMSC engraftment could regulate myogenic differentiation-related signaling pathway in the soleus up to 35 days after SNI.
MATERIALS AND METHODS
Experimental animals
Eight-five Sprague-Dawley rats (5 weeks old, male) were used for this experiment and they were divided with randomization method as follows: the normal control (CON, n=5), sedentary group (SED, n=20), low-intensity treadmill exercise group (TEX, n=20), BMSC transplantation group (BMSC, n=20), TEX+ BMSC transplantation group (TEX+BMSC, n=20) 7, 14, 21, and 35 days after SNI. Animals were housed in animal facility under 12/12-hr light-dark regime at 22°C and 60% of humidity, and fed commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. The use of rats in this study was approved by Ethical committee of Jeju National University (approval number: 2019–0026). And animal experiments were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals at Jeju National University.
BMSC culture
As described in study by Cho and Seo (2021), bone marrow tissues were collected by perfusion through femur and tibia of 4-week-old SD rats with the culture medium and they were cultured in Dulbecco’s modified Eagle’s medium with 20% fetal calf serum. Five to 7 days later, BMSCs adhered to the base on the culture dishes proliferated to a density of ca. 5×106 in one dish. Cells for transplantation were harvested by trypsin treatment followed by phosphate-buffered saline (PBS) washing.
SNI and BMSC transplantation
The surgical methods were fundamentally the same as those described in our previous paper (Cho and Seo, 2021; Seo et al., 2009). Briefly, rats were anesthetized by an animal inhalation narcosis control (Jeungdo Bio & Plant, Seoul, Korea). First, the rats were placed into the chamber with 2%–2.5% concentration of isoflurane for anesthesia and then 1.5%–1.8% concentration for maintenance during SNI. Sciatic nerve crush injury was applied into the middle of thigh twice for 1 min and 30 sec at interval (Seo et al., 2021). Single dose of 5×106 harvested BMSC (30-μL PBS) was injected into the injury area using a 30-gauge needle. After surgery, anesthetized animals were then placed on a heating pad maintained at 37°C, and then they were put in their cages for resting.
Treadmill exercise
All rats in this experiment had an adaptation period to treadmill exercise for 2 weeks. Animals in the exercise groups were rested for 2 days after SNI, and they started low-intensity treadmill exercise on third postoperation day. Low-intensity exercise on the treadmill device (Jeungdo Bio & Plant) was comprised of walking at a speed of 4 to 8 m/min for 30 min once a day from 7 days to 35 days postinjury.
Western blot analysis
Protein lysates were extracted from the dissected sciatic nerve tissues into triton lysis buffer, and the nucleus and cytoplasm were separated by nuclear extraction buffer and cytosol extraction buffer. Denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4°C. Protein (20 μg) was used for Western blot analysis using antiphosphorylated extracellular signal-regulated protein kinase 1/2 (ERK1/2) mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), antiphosphorylated p90 ribosomal s6 kinase (RSK) rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology, Danvers, MA, USA), antiphosphorylated mTOR rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), antiphosphorylated protein kinase B (Akt) rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-MyoD mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), antimyogenin mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-β-actin mouse monoclonal antibody (1:2,000, Santa Cruz Biotechnology), anti-GAPDH rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), and goat anti-mouse or goat anti-rabbit horseradish peroxidase conjugated secondary antibody (1:1,000, GeneTex Inc., Irvine, CA, USA) were used. The blotting proteins were detected by using Westar ECL substrates (Cyanagen, Bologna, Italy). Analysis of protein density was performed using Chemidoc (Bio-Rad, Hercules, CA, USA).
RESULTS
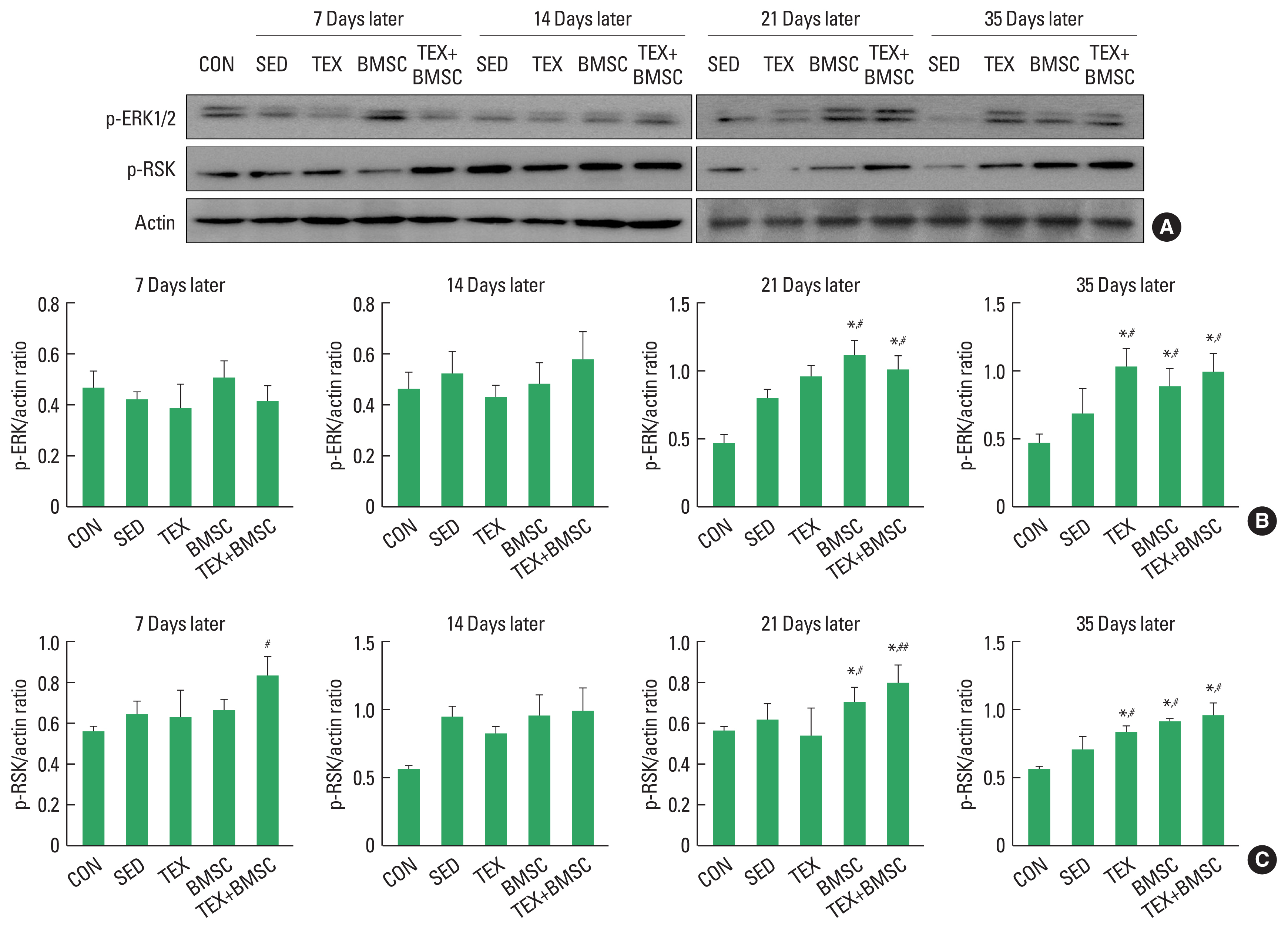
Time-dependent changes of regeneration proteins in the injured sciatic nerve
To confirm the time-dependent effect of combined low-intensity treadmill exercise and BMSC engraftment on expression levels of p-ERK1/2 and p-RSK, proliferative markers of Schwann cell, in the injured sciatic nerve, we performed biochemical experiment using Western blotting techniques. As shown in Fig. 1, p-ERK1/2 levels were increased until 21 days after SNI and then it was disappeared at day 35, whereas BMSC and TEX+BMSC group significantly upregulated p-ERK1/2 from day 21 to 35 compared to other groups (Fig. 1A, B). The effect of treadmill exercise applied alone after SNI was observed only at 35 days later. In addition, p-RSK was sharply increased until 14 days later and then rapidly downregulated, whereas TEX, BMSC, and TEX+BMSC groups continuously maintained activation of RSK until 35 days post injury compared to SED group (Fig. 1A, C).
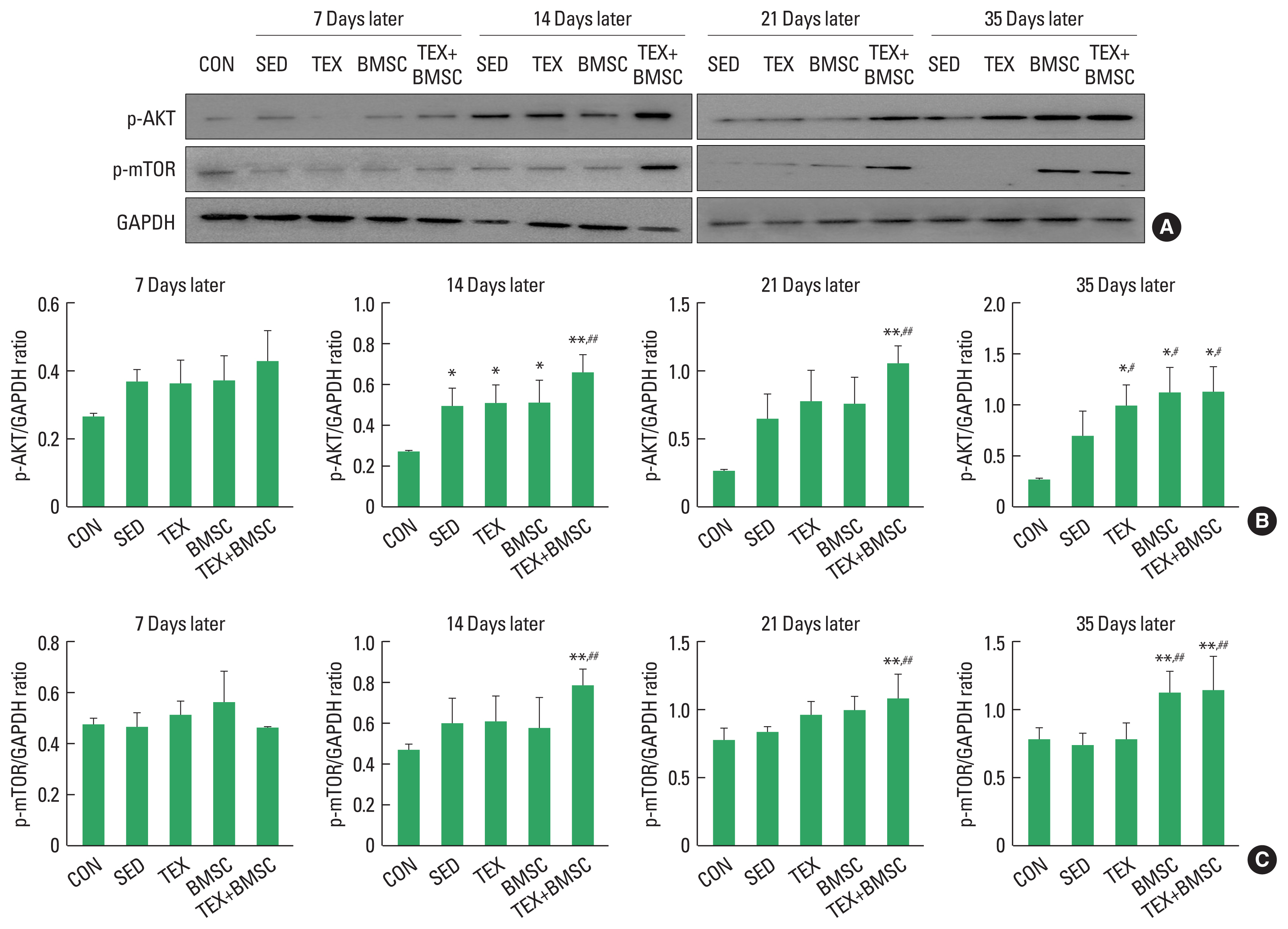
Time-dependent changes of hypertrophy-related proteins in the denervated soleus
To examine the time-dependent metabolic changes of hypertrophy in soleus muscle after SNI, we confirmed activation of Akt-mTOR molecular cascade using Western blotting. As shown in Fig. 2, p-Akt was expressed the highest on day 14 and showed a tendency to disappear from day 21, while TEX and BMSC groups continuously maintained expression level of p-Akt until day 35 (Fig. 2A, B). In particular, TEX+BMSC group significantly upregulated p-Akt from day 21 to day 35 compared to those in SED and TEX groups. p-mTOR protein was slightly induced in SED, TEX and BMSC groups at 7 and 14 days after SNI, whereas TEX+ BMSC group dramatically upregulated p-mTOR induction at day 14, and then continuously kept up expression levels until day 35 (Fig. 2A, C).
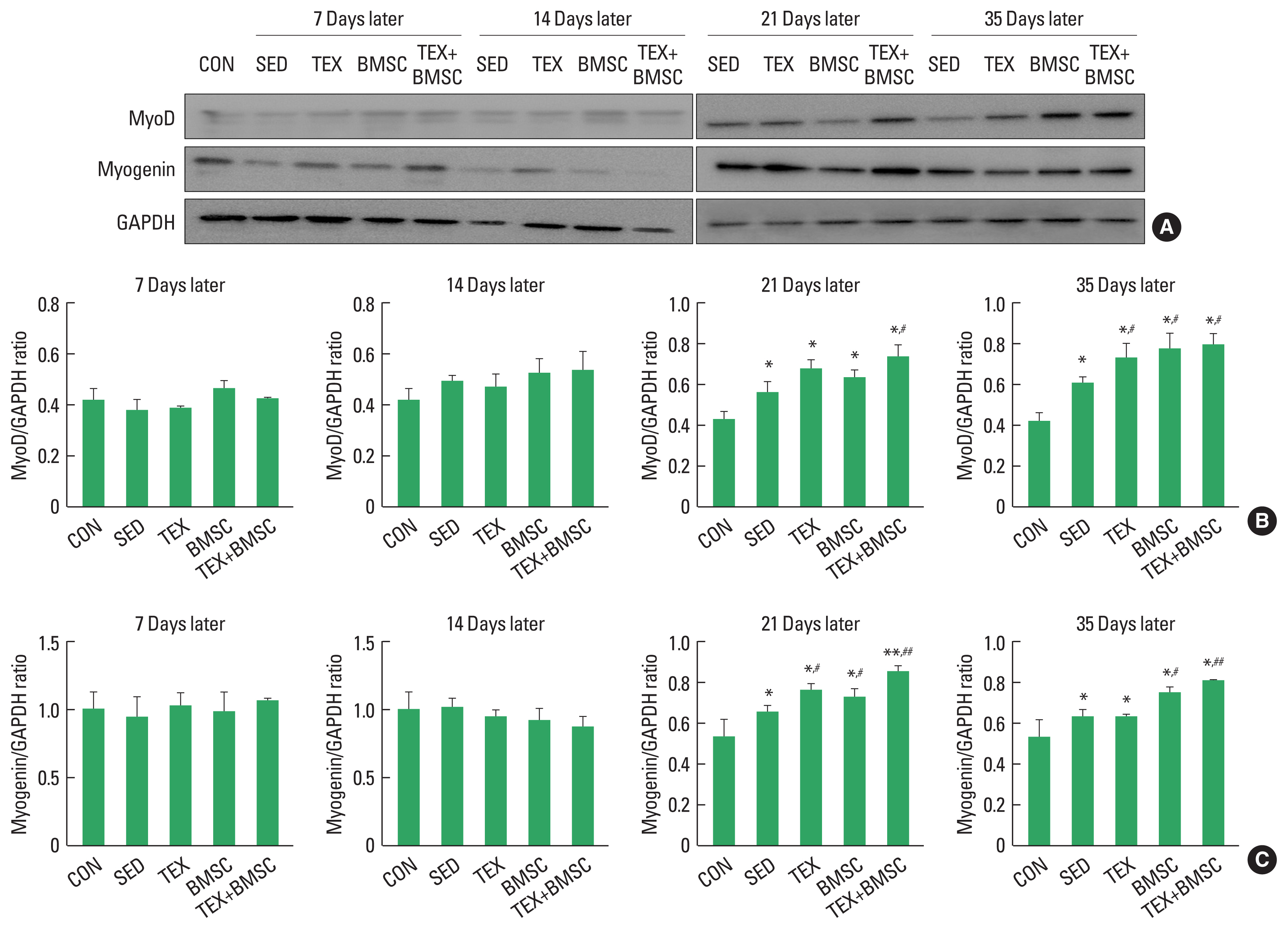
Time-dependent changes of myogenic differentiation-related proteins in the denervated soleus
To identify the time-dependent metabolic changes in myogenic differentiation of reinnervated soleus muscle, we confirmed phosphorylation of MyoD and myogenin proteins using Western blotting. As shown in Fig. 3, MyoD and myogenin did not change significantly in the reinnervated soleus muscle until 14 days after SNI, but they were meaningfully increased from 21 days later. TEX, BMSC, and TEX+BMSC groups also significantly enhanced expression levels of MyoD and myogenin from day 21 to day 35. In particular, MyoD and myogenin expression levels were increased the most in TEX+BMSC group than in other groups.
DISCUSSION
Immediately after SNI, Schwann cells undergo demyelination and dedifferentiation along with recruited macrophages and Wallerian degeneration is progressed. Over time, the proliferation of Schwann cells around the injury site becomes active, which is a starting signal for axonal regeneration of the injured sciatic nerve (Min et al., 2021). Mitogen-activated protein kinase (MAPK/ERK) pathway is a central regulator for proliferation and differentiation of Schwann cells in peripheral nerves and it has been known to response rapidly in the early stage of sciatic nerve regeneration (Wen et al., 2022). Also, inhibition of the RSK, downstream molecule of ERK1/2 protein, suppress the sprouting sciatic nerve fibers extending from dorsal root ganglion neurons (Boyette-Davis et al., 2015; Mao et al., 2022). In the present study, we examined time-dependent activation of ERK1/2 and RSK signaling pathway in the sciatic nerve up to 35 days after SNI, and confirmed that p-ERK1/2 was significantly increased in TEX group only at day 35 compared to the SED group, but BMSC and TEX+BMSC groups dramatically upregulated p-ERK1/2 expression from day 21 than those in the SED group and then maintained the induction levels until day 35. In addition, p-RSK was sharply increased 14 days later, and then rapidly downregulated from day 21, whereas TEX, BMSC and TEX+BMSC groups significantly kept up expression levels of p-RSK until 35 days post injury compared to SED group. In particular, concurrent application of exercise and BMSC engraftment critically accelerated the proliferation rate of Schwann cell at day 21 and 35. We believe that BMSC transplant treatment has a synergistic effect that further enhances the effect of aerobic exercise to promote regenerative protein expression. In previous studies, Seo et al. (2021) and Hu et al. (2016) reported that p-ERK1/2 was expressed from the early stage of the sciatic nerve regeneration to induce Schwann cell proliferation, and then disappeared a few days later as well as treadmill exercise could continuously upregulate p-ERK1/2 levels until 28 days after SNI. These time-dependent effect on activation of ERK1/2-RSK signaling support our findings that low-intensity exercise and BMSC transplantation might continuously maintain the induction of cell proliferation-related proteins until the late stage of sciatic nerve regeneration.
Damage of sciatic nerve that control skeletal muscles of the lower limbs is closely related with loss of muscle mass (atrophy) and this muscle atrophy results in behavioral dysfunction due to reduction of strength, endurance and energy metabolism in skeletal muscle (Grönholdt-Klein et al., 2019). Phosphorylation of mTOR and MRF signaling networks has been known as a specific regulator to prevent atrophy in the denervated muscles (Brooks and Myburgh, 2014), and these mechanisms is stimulated by anaerobic exercise and cell transplantation approaches applied during denervation period (Chen et al., 2022; Cho and Seo, 2021). Proteins-regulated kinase Akt is upstream protein to lead to activation of mTOR signaling pathway, whose downstream substrate including p70S6K not only promote protein synthesis but also inhibit muscle atrophy (Yin et al., 2020). mTOR is also upstream enhancer to regulate a subset of MyoD-regulated myogenic pathway (Ge and Chen, 2012; Yoon and Chen, 2013). Thus, we have identified time-dependent changes of Akr-mTOR-MyoD signaling network in the soleus muscle up to 35 days after SNI. At the initial period of nerve regeneration, p-Akt and mTOR were slightly increased and MyoD and myegenin was not induced as well as the application of aerobic exercise along after SNI did not result in dramatic hypertrophy-related protein expression. But TEX+ BMSC group significantly increased activation of Akt-mTOR in the reinnervated soleus from day 14 and MyoD-myogenin pathways was activated in BMSC+EX group from day 21. Sakakima and Yoshida (2003) suggested that the denervated muscle could speed up morphological recovery after the end of injured sciatic nerve axonal regeneration and Akt-mTOR signaling network absolutely contributed to hypertrophy of the denervated muscle. Previous studies on the role of the members of MRF family of transcription factors demonstrated that myogenin overexpression in mouse facilitated proliferating satellite cells to go through myogenic differentiation and muscle growth (Ganassi et al., 2020; Zammit et al., 2004), and MyoD is colocalized with myogenic factor 5 in satellite cells during the stage of skeletal muscle hypertrophy (Wang et al., 2022). These previous results implicate that combined intervention of aerobic exercise and BMSC transplantation might be a new therapeutic strategy for overcoming the morphological and functional problems in denervated soleus muscle.