INTRODUCTION
Damage to the sciatic nerve, a peripheral nerve in the lower human body, can result from conditions like traumatic nerve injury, nerve inflammation, and strenuous sports activities, leading to neuropathic pain (NP) and mechanical allodynia (Finnerup et al., 2021; Wong et al., 2022). NP is a well-known and very complex type of pain caused by traumatic nerve injury and dysfunction in the nervous system. It is accompanied by spontaneous pain (dysesthesia), pain sensations to nonpainful stimuli (allodynia), and increased burning pain response to painful stimuli (hyperalgesia) (Gilron et al., 2006; Hansson, 2003; McDermott et al., 2006). Also, NP results in loss of sensory neuron and function by releasing inflammatory cytokines such as tumor necrosis factor α (TNF-α), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interleukin (IL) (Sweitzer et al., 2001; Zelenka et al., 2005). It has been reported that the occurrence of NP adversely affects quality of life, and imposes a substantial economic burden (Berger et al., 2004).
Nociceptors are found in all areas of the body, including the skin, muscles, joints, and digestive system. The dorsal root ganglia (DRG), which function as sensory processing organs, transmit sensation from the skin to the brain and play a crucial role in transmitting inflammation and pain, particularly after peripheral nerve damage (Deer et al., 2013; Krames, 2014). Various therapeutic strategies have been employed to alleviate NP by targeting DRG neurons (DRGs). In the medical field, electrical neuromodulation therapy, drug therapy, and surgical interventions have been shown to be effective (Liem et al., 2013; Pope et al., 2013). However, these approaches face challenges concerning economic costs and side effects, and NP remains a persistent and significant neurosurgical problem (Magrinelli et al., 2013).
As the most economical therapeutic method to improve NP and inflammation targeting the injured DRG, the application of mild exercise in early stage of nerve regeneration has long been recommend (Cooper et al., 2016; Detloff et al., 2014). Regular exercise has a positive effect on antinociceptive effects, nerve regeneration, neuroplasticity, and changes in messenger ribonucleic acid expression related to cell death (Galdino et al., 2014; Keeler et al., 2012; Molteni et al., 2004). Additionally, endurance and resistance exercise decrease proinflammatory cytokines and increase anti-inflammatory markers (Gleeson et al., 2011).
Nevertheless, there is a lack of clear evidence demonstrating that exercise can modulate the expression of NP and inflammatory cytokine markers, as well as improve NP in the early stages of nerve regeneration after sciatic nerve injury (SNI). Therefore, the purpose of this study is to investigate whether walking exercise can regulate the expression levels of NP and inflammatory markers in the ipsilateral lumbar 4 (L4) to 6 (L6) DRGs during the early stages of nerve regeneration after SNI.
MATERIALS AND METHODS
Experimental animals
This experiment used male Sprague-Dawley rats aged 5 weeks. The experimental rats were randomly divided into seven groups: the normal control group, sedentary groups for 3, 7, and, 14 days postinjury (dpi), and walking exercise groups for 3-, 7-, and 14-dpi. The animals were maintained under a constant room temperature of 22°C–24°C and 60% humidity, with a 12/12-hr light-dark cycle. They were provided with commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. This experiment was performed according to the guidelines of the approved Institutional Animal Care and Use Committee protocol (protocol number: 2022-0028) from Jeju National University.
Sciatic nerve injury protocol
In the injury groups, rats were anesthetized using an animal inhalation narcosis control (Jeungdo Bio and Plant, Seoul, Korea). The rats were placed into the chamber with a 2%–2.5% concentration of isoflurane for anesthesia, and then maintained at 1.5%–1.8% concentration during sciatic nerve injury. The left sciatic nerve was exposed and crushed with a pair of forceps held tightly for 1 min and 30 sec at intervals (Yu et al., 2023). After the surgery, the anesthetized animals were placed on a heating pad maintained at 37°C and then returned to their cages for resting.
Walking exercise protocol
To adapt the walking exercise, all rats participated in this study were subjected to low-intensity walking exercise for a week before the study began. Rats in the exercise groups performed walking exercise on the treadmill device (Jeungdo Bio and Plant) at 8 m/min for 30 min with no inclination during the experiment duration (3, 7, and 14 days). The walking exercise was performed after resting for 2 days, excluding the control and sedentary groups.
Western blot analysis
The dissected L4 and L6 DRGs tissues were rinsed with phosphate-buffered saline and lysed in Triton lysis buffer. Denatured proteins were then separated on a sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. Subsequently, the membranes were blocked with 5% skim milk and 0.1% Tween 20 in tris-buffered saline for 30 min at room temperature. Following this, the membranes were incubated overnight with primary antibodies at 4°C. For the Western blot analysis, 20 μg of protein was used, and the following primary antibodies were applied: antigrowth associated protein 43 (GAP-43) mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti–brain-derived neurotrophic factor (BDNF) mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti–NF-κB mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti–TNF-α rabbit polyclonal antibody (1:1,000, Sino Biological, Wayne, PA, USA), and anticalcitonin gene-related peptide (anti-CGRP) mouse monoclonal antibodies (1:1,000, Santa Cruz Biotechnology), and anti-c-Fos mouse monoclonal antibody (1:1,000, Abcam, Cambridge, UK), and anti-IL-6 rabbit polyclonal antibody (1:1,000, GeneTex Inc., Irvine, CA, USA), anti-β-actin mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (1:1,000, GeneTex) were used as the secondary antibodies. The blotting proteins were detected using Westar ECL substrates (Cyanagen, Bologna, Italy), and the detected band intensity was analyzed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Paw withdrawal test
For the assessment of mechanical allodynia, rats were placed individually in clear boxes with holes, allowing them to adapt for 30 min before the test. The von Frey filament (BIO-EVF4, Bioseb, Vitrolles, France), consisting of plastic hairs with calibrated diameters, was applied to the plantar surface of the left hind paw using a series of ascending forces (Chaplan et al., 1994). Each rat was tested 3 times, and the time and intensity of hind paw withdrawal were measured. The filaments with calibrated forces of 0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, and 10 g were most frequently used. The von Frey test was performed before the injury and 3, 7, 10, and 14 dpi.
Statistical analysis
All the data are presented as the mean±standard error. Statistical analysis was performed using one-way analysis of variance, followed by the Duncan post hoc test. The significance level was set at P<0.05. Data analysis and graphs were generated using Prism 6 software (GraphPad, La Jolla, CA, USA).
RESULTS
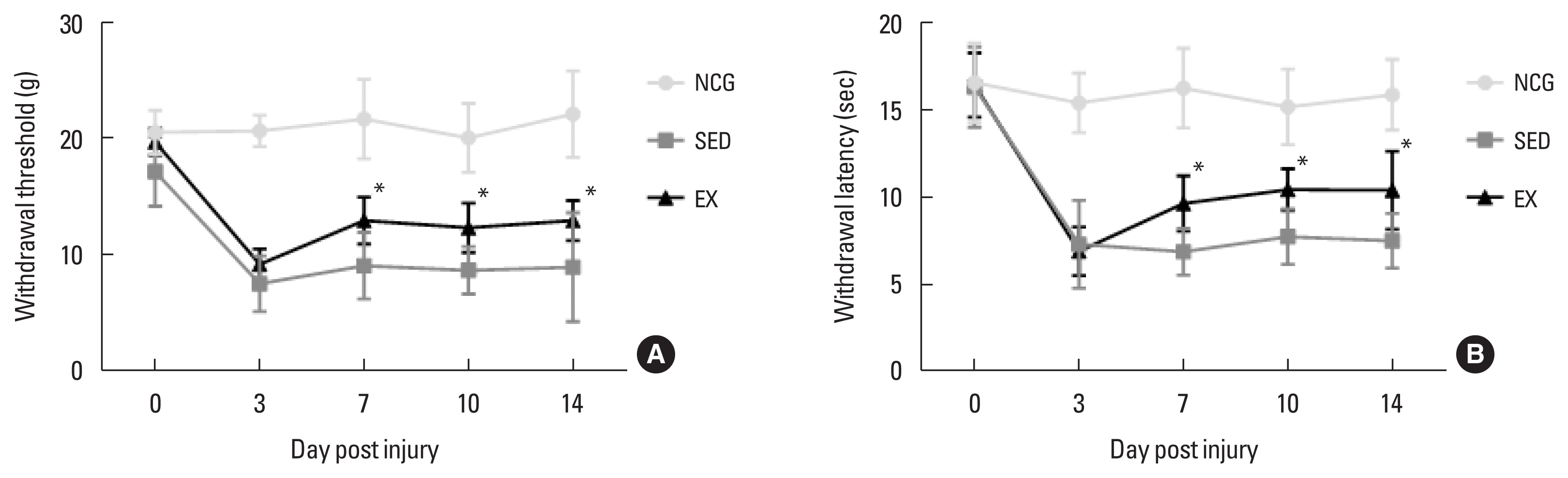
The walking exercise improved mechanical allodynia after SNI
To confirm the effect of walking exercise on peripheral pain-like behavior after SNI, we analyzed the time-dependent effect on latency for hind paw withdrawal using the von Frey test device. As shown in Fig. 1, mechanical withdraw threshold and latency in hind paw withdrawal test (grams and time) were significantly improved in the walking exercise group compared to the sedentary group at 7 dpi (P<0.034, P<0.037), 10 dpi (P<0.046, P<0.044), and 14 dpi (P<0.027, P<0.028). However, no significant difference was observed between the exercise and sedentary groups at 3 dpi (P<0.518, P<0.931).
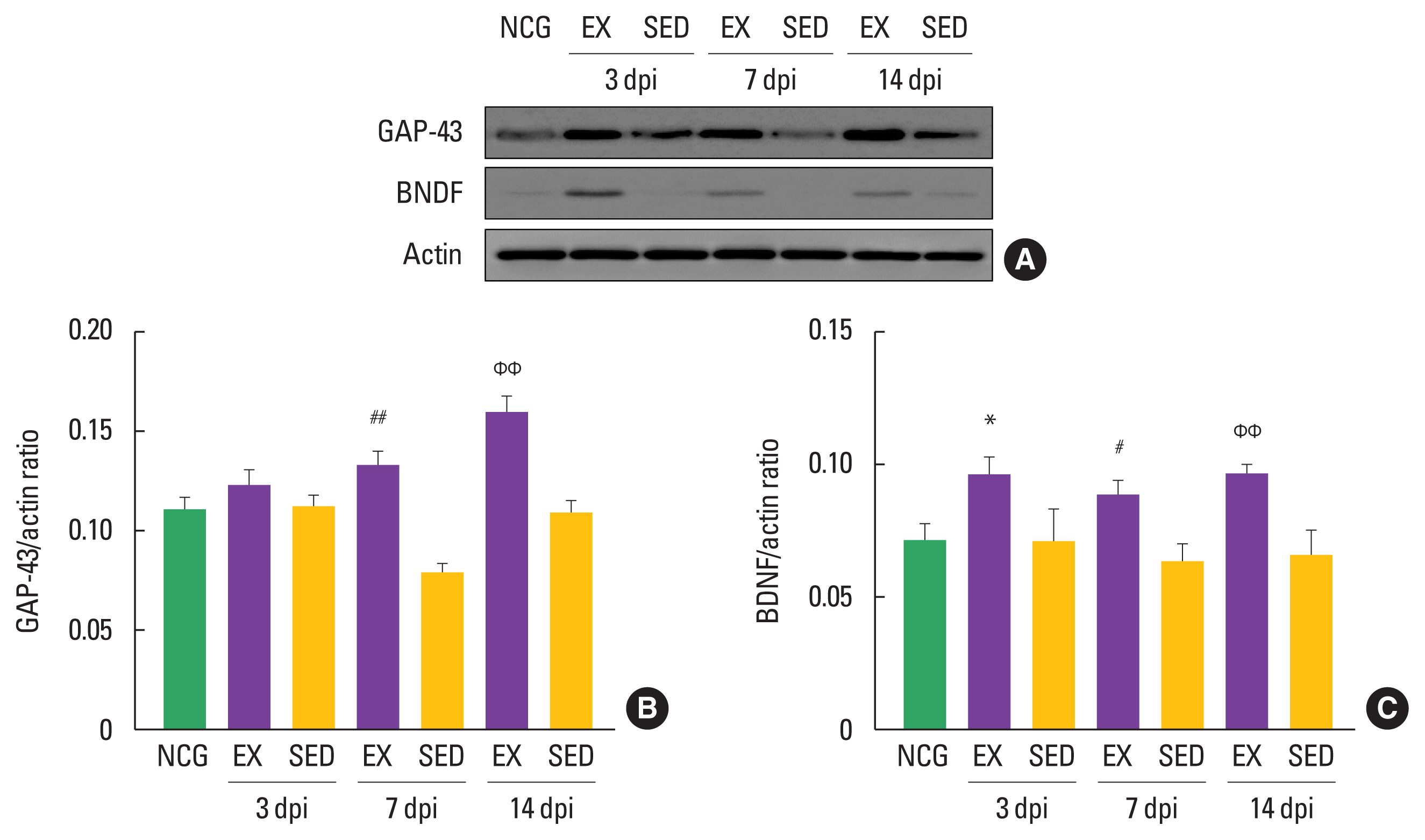
The walking exercise increased induction of nerve regeneration proteins in the in ipsilateral DRGs
To examine the timing-dependent effect of walking exercise on nerve regeneration-related markers in the ipsilateral DRGs after SNI, we analyzed the expression levels of GAP-43 and BDNF using Western blot techniques. As shown in Fig. 2, GAP-43 was significantly increased in the walking exercise group compared to the sedentary group at 7 dpi (P<0.01), and 14 dpi (P<0.01), However, there was no significant difference at 3 dpi (P<0.42). The walking exercise was upregulation BDNF protein compared to the sedentary group at 3 dpi (P<0.014), 7 dpi (P<0.02), and 14 dpi (P< 0.004), suggesting that walking exercise at each time point after SNI may be a therapeutic strategy for sciatic nerve regeneration.
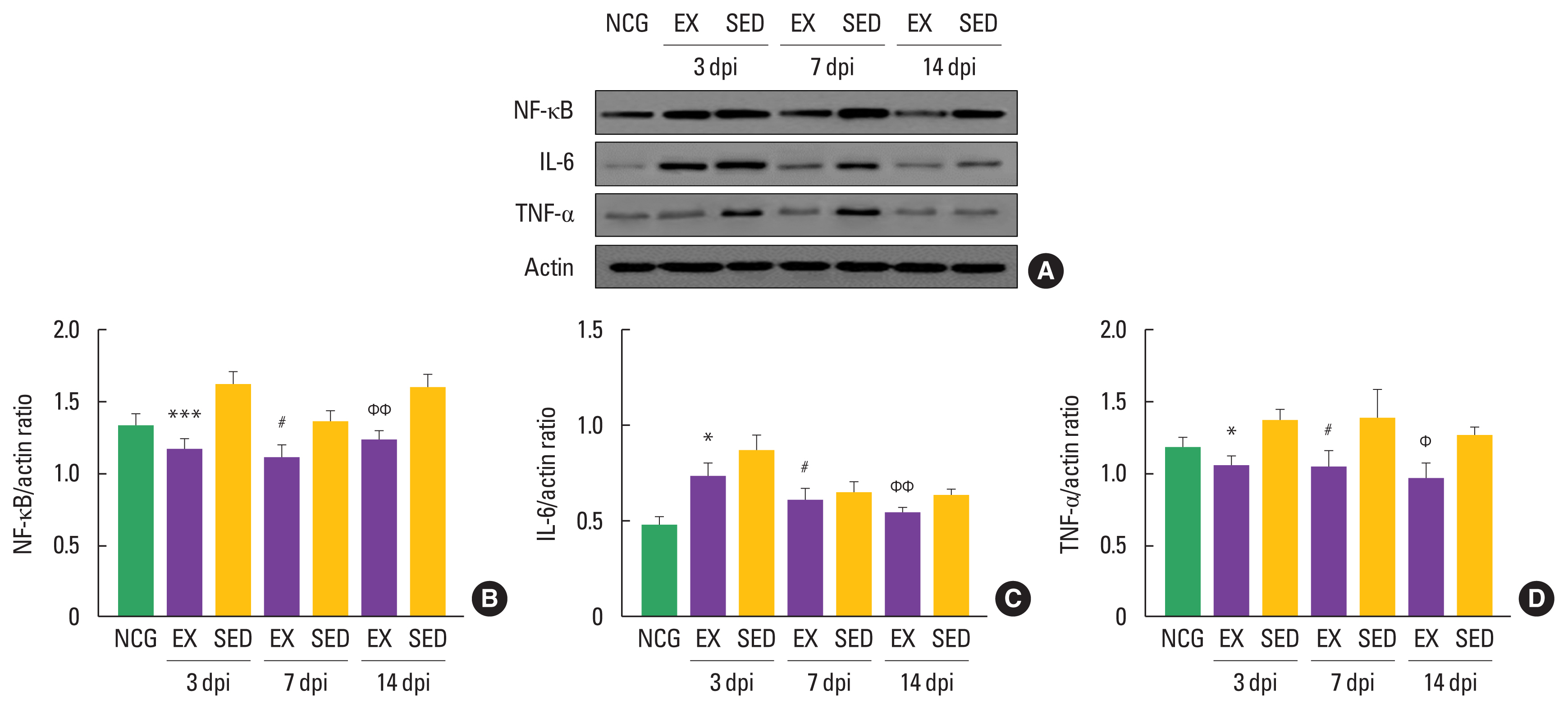
The walking exercise decreased levels of NP proteins in the in ipsilateral DRGs
NP-related markers, such as NF-κB, TNF-α, and IL-6, have been known to be activated in DRGs after denervated sciatic nerve. To confirm the timing-dependent effect of walking exercise on these markers in the ipsilateral DRGs after SNI using Western blot techniques, we analyzed their expression levels. As shown in Fig. 3, NF-κB was decreased in the walking exercise group compared to the sedentary group at 3 dpi (P<0.001), 7 dpi (P<0.019), and 14 dpi (P<0.009). Walking exercise was significantly upregulated IL-6 expression level than those the sedentary group at 3 dpi (P<0.011), 7 dpi (P<0.011), and 14 dpi (P<0.005). TNF-α was decreased in the walking exercise group compared to the sedentary group at 3 dpi (P<0.012), 7 dpi (P<0.018), and 14 dpi (P< 0.012), suggesting that walking exercise after SNI may be a positive modulator for NP.
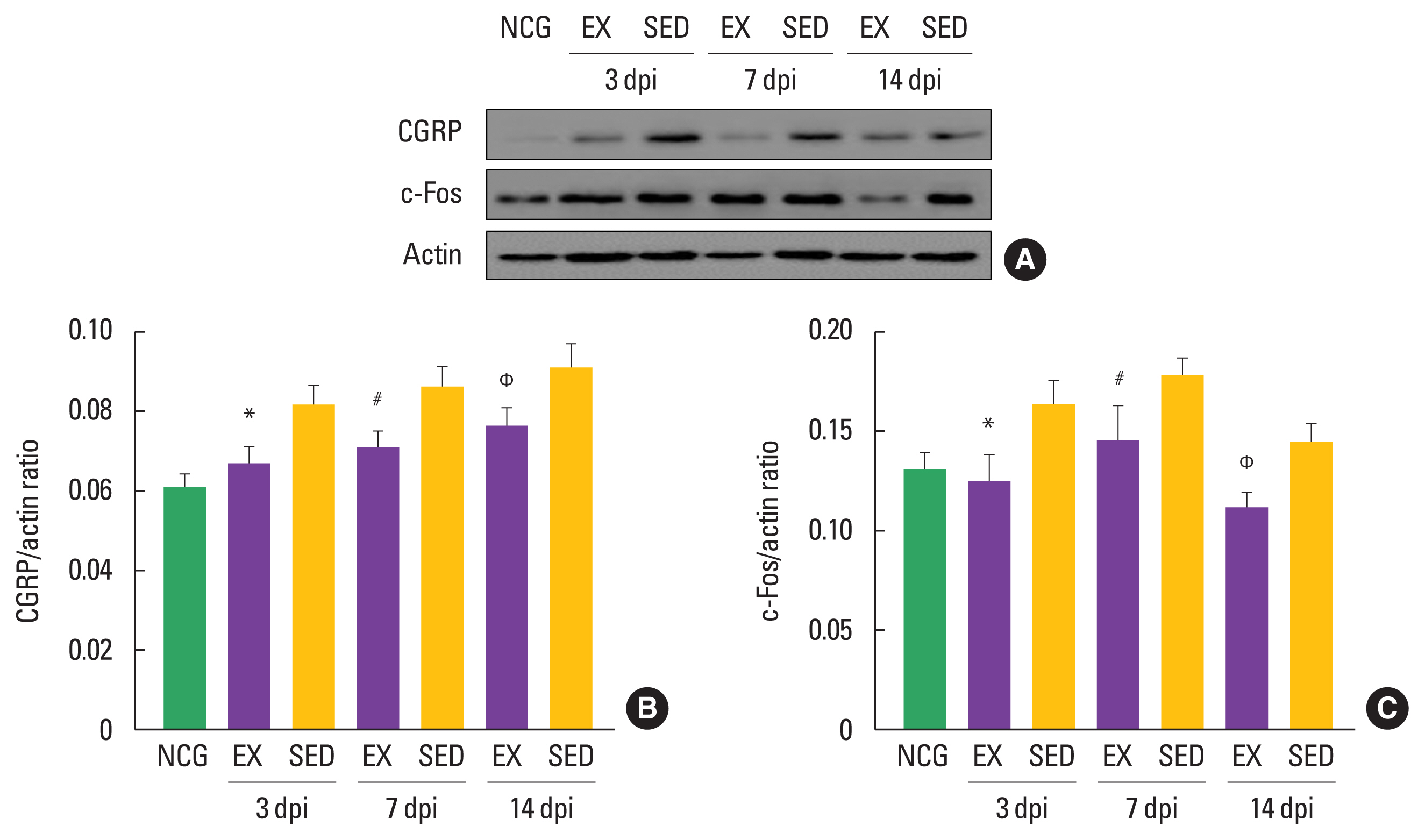
The walking exercise decreased induction of inflammatory markers in the in ipsilateral DRGs
To confirm the timing-dependent effect of walking exercise on CGRP and c-Fos expression levels in the ipsilateral DRGs after SNI using Western blot techniques, we analyzed their expression levels. As shown in Fig. 4, CGRP was decreased in the walking exercise group compared to the sedentary group at 3 dpi (P<0.018), 7 dpi (P<0.012), and 14 dpi (P<0.022). Walking exercise was significantly downregulated c-Fos expression level than those the sedentary group at 3 dpi (P<0.013), 7 dpi (P<0.042), and 14 dpi (P<0.041), suggesting that walking exercise after SNI might be a strategy to improve the inflammatory response in ipsilateral DRGs.
DISCUSSION
Damage to peripheral nerves can indeed lead to a variety of physiological, behavioral, and morphological changes from the injury site to distal part. These changes include Wallerian degeneration, denervation, and muscle atrophy (Scheib and Höke, 2013). Additionally, nerve damage results in increased mechanical allodynia and NP. Previous studies have reported chronic exercise during regeneration period might alleviate NP and promote functional recovery after SNI. But the exact mechanism remains unclear. Therefore, this study investigated changes in inflammatory and NP in the early stage of sciatic nerve regeneration by using Western blot and the von Frey test device.
Peripheral nerve damage is known to cause allodynia rather than hyperalgesia, and the von Frey test is the most reliable test method to confirm mechanical allodynia by nerve damage (Hogan et al., 2004; Lambert et al., 2009). We found that walking exercise was significantly alleviated mechanical allodynia compared to sedentary group at the 7, 10, and 14 dpi. Regular exercise over 14 days reduced both mechanical allodynia and thermal hyperalgesia after nerve injury in rats (Shen et al., 2013). However, another study reported that voluntary exercise training did not improve symptoms of mechanical allodynia in nerve-injured states (Sheahan et al., 2015). The different results are thought to be due to differences in the SNI method and exercise type.
For decade, GAP-43 and BDNF has been well known to facilitate neurite outgrowth in denervated peripheral nerves (Lopes et al., 2017). We confirmed that walking exercise significantly upregulated GAP-43 and BDNF expression levels at all time points (3, 7, and 14 dpi) compared to the sedentary groups. Previous studies have reported that Schwann cell proliferation is a series of processes that occur in the early stage of sciatic nerve regeneration, and GAP-43 and BDNF induction levels are upregulated by aerobic and anaerobic exercise for Schwann cell proliferation after SNI (Chiaramonte et al., 2023; Udina et al., 2011). Another study reported that over 4 weeks of exercise could be positive on sciatic nerve regeneration by upregulation GAP-43 (Arabzadeh et al., 2022). The results of previous studies support our findings that walking exercise might effectively increase nerve regeneration markers, including GAP-43 and BDNF in the early stage of regeneration after SNI.
Recent studies on NP have confirmed increase of cytokines in the DRG or spinal cord after nerve injury (Clark et al., 2013), suggesting the importance of blocking the proinflammatory cytokines such as TNF-α, NF-κB, IL-6, and IL-1β to minimize NP after nerve injury (Sacerdote et al., 2013). Thus, we investigated expression levels of NP makers in the ipsilateral DRGs and confirmed that exercise sharply downregulated NF-κB, TNF-α, and IL-6 levels compared to the sedentary groups at all time points (3, 7, and 14 dpi). Previous studies emphasized that regular swim or running exercise might have a regulation of NP and proinflammatory cytokines proteins (IL-6, TNF-α, NF-κB) (Bobinski et al., 2011; Chen et al., 2012). These findings in previous studies support our findings that regular walking exercise might be an importance mechanism for down-regulation of NP and proinflammatory cytokines in the injured sciatic nerve.
CGRP, an indicator for NP and inflammation, has been proposed contribute to nociceptive pathways in both central and peripheral nerves in the humans. And c-Fos have been strongly linked to inflammatory and nociceptive response to injured nervous system (Schou et al., 2017; Tabata et al., 2018; Terayama et al., 2023). A recent study reported that CGRP in the injured sciatic nerve were downregulated during 3 weeks of voluntary wheel running exercise (Cheng et al., 2022). Another study demonstrated that treadmill exercise for 4 weeks reduced the level of c-Fos expression in the dorsal raphe nucleus (Nishii et al., 2017). These previous studies imply that the expression of CGRP and c-Fos can be regulated through chronic exercise, although it is not changed in DRGs. Our study found that participation in walking exercise suppressed injury-induced CGRP and c-Fos expression levels in the ipsilateral DRGs compared to the sedentary group at all time points.
Considering all these results, our findings suggest critical information that aggressive rehabilitation in the early stage of regeneration after SNI might improve NP and inflammatory cytokines. However, the present study has the limitation of observing short-term effect of exercise on NP after SNI. Therefore, further research is necessary to analyze the effect of long-term period exercise on NP-related proteins after SNI.