Changes in training posture induce changes in the chest wall movement and respiratory muscle activation during respiratory muscle training
Article information
Abstract
Postural changes induce changes in chest wall kinematics and eventually pulmonary function, and affect chest wall shape and chest motion. This study aimed to examine the effects of postural change on changes in the chest wall during respiratory muscle training. Using a repeated measures design, this study followed 13 healthy adults (13 men; mean age, 23.73 years). All participants performed four postures (neutral, full trunk rotation, half-range trunk rotation, and lateral ribcage shift postures) during respiratory muscle training. The chest wall movement during the four postures was measured using a three-dimensional motion-analysis system during respiratory muscle training. Surface electromyography data were collected from the diaphragm and sternocleidomastoid muscles, and the asymmetric ratio of muscle activation was calculated based on the collected data. The chest wall movements of the upper costal and middle costal region were greater in the neutral posture than in the full rotation, half rotation, and lateral ribcage shift postures (P<0.05). The respiratory muscle activation on diaphragm of left was greater in the full rotation posture than in the neutral posture, half rotation, and lateral ribcage shift postures (P<0.05). The asymmetric ratio of muscle activation was greater in the full rotation posture than in the neutral posture, half rotation, and lateral ribcage shift postures (P<0.05). This study verified that postural change during respiratory muscle training may affect chest wall movement and muscle activation. Thus, this study recommends respiratory muscle training to be performed in neutral posture.
INTRODUCTION
Postural change leads to changes in spontaneous quiet breathing and affects thoraco-abdominal kinematics. The interaction among posture, patient sex, and rib cage and abdominal kinematics during quiet breathing in turn has an impact on chest wall kinematics (Romei et al., 2010; Sharp et al., 1975; Verschakelen and Demedts, 1995).
Two studies have reported that changes in body position alter pulmonary functions, and they have reported on the relative contributions of the rib cage and abdomen to ventilation (Romei et al., 2010; Sharp et al., 1975). Postural changes have been noted to impact chest wall shape, motion, and motion distribution between compartments of the chest wall (Lee et al., 2010). Furthermore, thoracic rotation has been reported to decrease rib cage motion by altering rib articulations, the required intercostal muscle activity, and abdominal displacement (Lee et al., 2010; Lin et al., 2006).
Based on this theory, previous studies have confirmed the effects of postural change during quiet and deep breathing on chest wall volume and right and left chest volume (Aliverti et al., 2001), as well as a high correlation between postural changes and changes in vital capacity and quiet breathing (Verschakelen and Demedts, 1995). Previous studies have analyzed the effects of posture on chest wall kinematics and lung volume in various postures, including the supine and prone positions and a shift from the seated position to the supine position (Aliverti et al., 2001; Romei et al., 2010). In addition, the changes of chest wall volume in the lateral position have also been investigated (Nozoe et al., 2014). However, the changes in chest wall kinematics during the thoracic rotation and side-bending postures have not been compared. The thoracic rotation and side-bending postures are commonly utilized for partial expansion of the chest wall and chest mobilization in the clinical setting during training (Leelarungrayub et al., 2009).
Several portable respiratory muscle training devices have been developed in the last 20 years that are aimed to help patients with respiratory, cardiac, or neurological disorders to minimize or revert these alterations (Lima et al., 2014; McConnell and Romer, 2004). Among these devices, incentive spirometers and inspiratory muscle threshold trainers preserve airway patency by increasing respiratory muscle activity, such as the patients’ diaphragm muscle and external intercostal muscles; they also act to increase the volume of the thoracic cavity, which forces air into the lungs (Xiao et al., 2012).
Recent reports have investigated the changes in chest wall volume in patients via chest wall kinematic analysis using respiratory muscle training devices and compared changes between the right and left chest wall volume. Through their analysis, the authors suggested that respiratory muscle training devices can enhance chest wall volume and reduce asymmetry (Lima et al., 2014). However, chest kinematic analyses that document the degree of expansion of specific areas of the chest wall through postural changes during respiratory muscle training have not been attempted.
Thus, this study aimed to examine the effects of postural change during respiratory muscle training on changes of the chest wall movement and respiratory muscle activation.
MATERIALS AND METHODS
Participants
Thirteen men with normal pulmonary function participated in the study. The subjects were screened according to the following inclusion and exclusion criteria. The inclusion criteria were: (a) absence of cardiac and pulmonary disease, (b) nonsmokers, (c) no endurance-trained athletes, and (d) age older than 18 years (Romei et al., 2010). The exclusion criteria were (a) subjects with neurological findings who had undergone surgery, (b) those currently receiving surgical treatment, and (c) those taking pain medications on a regular basis (Jung and Kim, 2016). The subjects’ demographic characteristics are shown in Table 1.
All participants voluntarily provided consent to participate in this study. All protocols were approved by the Ethics Committee of the Catholic University of Pusan (CUPIRB-2015-008).
Procedure
Before testing, pulmonary function (forced vital capacity, forced expiratory volume in 1 sec, peak expiratory flow, and maximum inspiratory pressure [MIP]) was measured by using a spirometer (Pony Fx, Cosmed, Rome, Italy), and MIP was measured by using the Power Breathe K5 device (Power Breathe International Ltd., Warwickshire, UK).
Three-dimensional (3D) chest wall movement was measured and analyzed for four sitting positions: neutral, full trunk rotation to the left, half-range trunk rotation to the left, and lateral ribcage shift to the left posture (Kaneko and Horie, 2012; Lee et al., 2010) (Fig. 1).
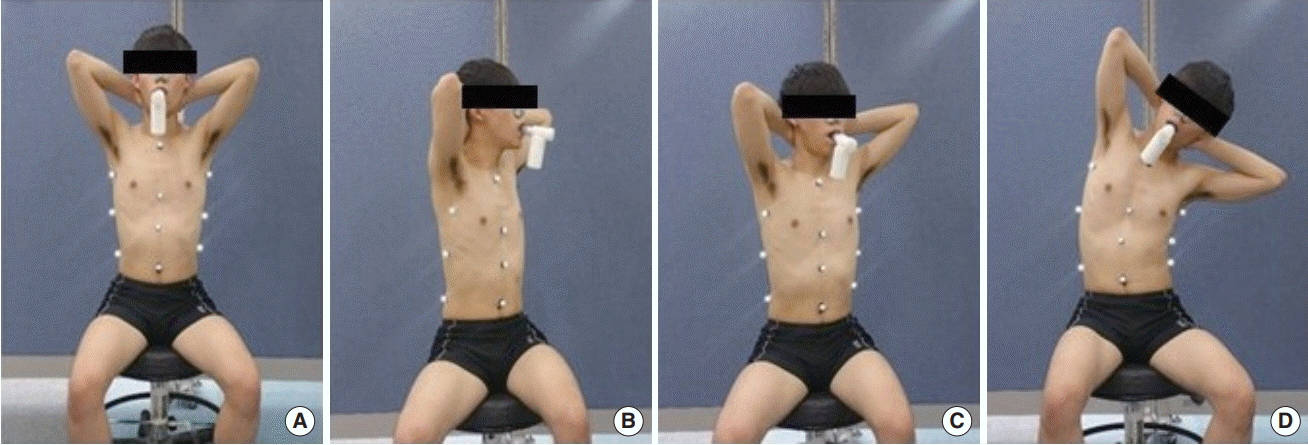
Four training posture. (A) Neutral posture, (B) full trunk rotation to the left posture, (C) half-range trunk rotation to the left posture, and (D) lateral ribcage shift to the left posture.
Test orders were randomly assigned and each subject was asked to select a card. Then, applied to the subjects in order from the card marked as one of the postures (Park et al., 2013). Once the testing began, the subjects wore a nose clip and respiratory muscle training device (Power Breathe medic classic; Power Breathe International Ltd., Warwickshire, UK) and breathed quietly. They performed the next stage of maximal inspiration through a mouthpiece until the respiratory valve was opened and air was let in, as instructed (Xiao et al., 2012). At each of the data collection points, maximal inspiratory efforts of 2- to 3-sec duration were performed 3 times in each posture, with an interval of at least 10 min between the postures. The chest wall movement and muscle activity outcomes were assessed concurrently (Hawkes et al., 2007; Paisani Dde et al., 2013).
Inspiratory muscle loading
A respiratory muscle training device was used to provide an acute bout of inspiratory muscle loading. The intensity was adjusted from the protocol used in a previous study (Hawkes et al., 2007), in which maximal inspiration at intensity of 30% of the MIP was measured prior to the experimental procedure (de Andrade et al., 2005).
Chest wall movement
A 3D motion-capture system (Oxford Metrics, Ltd., Oxford, UK; sampling rate 200 Hz) was used to measure the amount of chest wall movement during respiratory muscle training. Fourteen sensors were placed over landmarks on the upper, middle, and lower ribcage and abdomen to measure chest wall movement (Lee et al., 2010) (Fig. 2).

(A) Schematic diagrams showing marker positioning on the chest wall and positions of electrode placement on the sternocleidomastoid (SCM), diaphragm (DI) of the subject. (B) Line drawings of diameters calculated from sensor position. Manubrium sensor (Man), sternum sensor (St), axilla sensors on the 4th rib (AxL, AxR), 9th rib sensors (9R, 9L), vertebrae sensor (T7, T12P, L3), anterior lower costal sensor on T12 level (T12A), abdominal sensor (AbR, AbL), umbilicus sensor (Umb).
The kinematic data were analyzed using Vicon Nexus software ver. 1.5.2 (Vicon Motion Systems Ltd., Oxford, UK). The mean value of the diameter of the sensors from three trials was used for the analysis (Lee et al., 2010). Chest wall movement was the change in these diameters from the end of inspiration of to the end of expiration (Lee et al., 2010). The end of inspiration and the end of expiration were determined in consideration of the distances between the sensors in each area with reference to the point at which the sum of the lateral diameter and anteroposterior diameter of the middle ribcage region reached maximum and minimum, respectively.
Electromyographic analysis of the diaphragm and sternocleidomastoid
The electromyographic (EMG) activities of the diaphragm and sternocleidomastoid on both sides were measured with a surface EMG system (Delsys Trigno Wireless EMG System; Delsys, Inc., Boston, MA, USA) with a sampling rate of 2,000 Hz and bandwidth of 20–450 Hz, obtained with chest wall movement analysis (Hawkes et al., 2007; Kang et al., 2015; Lima et al., 2014). All data were converted to root-mean-square (RMS) values.
To minimize skin impedance, the skin with shaving any hair and the skin swabbed with alcohol cotton before the electrodes were placed. Each EMG probe was attached to two superficial reusable bipolar electrodes consisting of Ag/AgCl material and a conductive hydrogel adhesive. The electrodes were placed 2 cm apart from each other (Lima et al., 2014). The electrodes were placed on the diaphragm at the lowest intercostal spaces on both sides of the body and at the midclavicular line, and the sternocleidomastoid electrode was placed on the muscle body, 5 cm from the mastoid process (Hawkes et al., 2007; Lima et al., 2014) (Fig. 2). The diaphragm and sternocleidomastoid RMS values that were obtained via this method were entered into the following equation. A higher absolute value indicated a higher asymmetry ratio (Hsu et al., 2003). Asymmetry ratio=(1-[left side muscle RMS value/right side muscle RMS value]).
Statistical analysis
The sample size was calculated according to the data collected from 5 volunteers during respiratory muscle training. Considering a significance level of 0.05 and a statistical power of 0.80, the optimal number of subjects in the experiment was estimated as 10. A distribution test was performed and the data showed normal distribution. One-way repeated-measures analysis of variance (ANOVA) of within factors and post hoc Fisher least-significant-difference (LSD) tests were conducted for each chest wall movement in the four conditions and one-way ANOVA and post hoc LSD tests were conducted for the amount of muscle activation (RMS) in the four conditions. The partial eta-squared values were included as an indicator of effect size when using ANOVA. The data collected were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Statistical significance was set at P-value of 0.05.
RESULTS
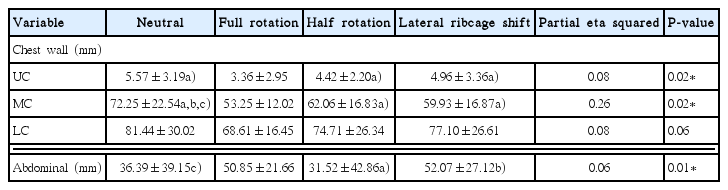
Significant differences were found in chest wall movement in the upper costal region during the neutral, half rotation, lateral ribcage shift postures, and full rotation posture between conditions (P<0.05) (Table 2). Significantly greater chest wall movement was observed in the middle costal region in the neutral posture than in the full rotation, half rotation, and lateral ribcage shift postures (P<0.05) (Table 2). Significant differences were found in chest wall movement in abdominal movement during the neutral, half rotation, lateral ribcage shift postures and full rotation posture between conditions (P<0.05) (Table 2).
Significant differences were found in muscle activation in the left diaphragm and asymmetry ratio of muscle activation in diaphragm during the neutral, half rotation, lateral ribcage shift postures, and full rotation posture between conditions (P<0.05) (Table 3).
DISCUSSION
In this study, we hypothesized that postural change during respiratory muscle training will alter chest wall expansion, and we attempted to verify the optimal posture for increasing chest wall mobility during respiratory muscle training by analyzing four training postures that were suggested by a previous study (Lee et al., 2010). Existing studies on respiratory muscle training have mostly focused on improving pulmonary function through training intensity, training periods, and training (Pollock et al., 2013; Xiao et al., 2012). However, the authors of the previous studies predicted that the training posture would affect overall chest wall mobility and muscle activation, eventually leading to changes in pulmonary function, which supports the findings of the current study.
Minor changes in posture have been reported to alter muscle activation and joint orientation to preserve continuous respiratory function, and the altered chest wall mobility in specific areas manifests as limited overall chest wall mobility or problems with flexibility of the respiratory apparatus (Lee et al., 2010). Furthermore, changes in postural alignment have been found to alter range of movement and affects coupling patterns of thoracic spine and rib articulations, subsequently inducing changes in articular movement for respiration (Edmondston et al., 2007).
The present study found evidence supporting that of previous studies. In all areas on the chest wall (the upper, middle, and lower costal regions), the neutral posture was associated with the highest chest wall mobility, while the full rotation posture led to limited chest wall mobility. This might be due to the fact that the ribcage becomes impacted as the trunk rotates around the spine and articular motion decreases during respiration, reducing chest wall compliance (Lee et al., 2010; Lee, 2015). Moreover, in the result of the analysis of each segment of the chest wall, the full rotation posture showed more limitations in movement in the upper and middle costal regions than the other postures. Meanwhile, the upper and middle costal regions were limited, with relatively higher movement in the lower costal region than in the lateral ribcage shifting posture, which was similar to the movement in the neutral posture. The limited movement in the middle costal region during the full rotation posture may be explained by biomechanical data in which the midthorax (T3–6) primarily contributes to spinal rotation (Panjabi, 1992). Although the lateral ribcage shifting posture, in which the trunk is laterally bent at the eighth rib, limited the movement of the upper and middle regions of the chest wall, it showed similar movement as the neutral posture, as there was free chest wall movement in the lower costal region below the ninth rib. Although there were no significant differences in the lower costal region movements between the lateral ribcage shifting posture and full rotation posture, these results imply that postural change is involved in expanding specific regions of the chest wall.
Adjusted posture alters trunk muscle activity and affects ribcage and abdominal compliance (Callaghan and Dunk, 2002; Lee et al., 2010; O’sullivan et al., 2002). Previous studies have reported that trunk rotation stimulates activity in the intercostal and abdominal muscles of both sides of the body (Andersson et al., 2002; Rimmer et al., 1995), and that the ribcage and abdominal compliance are affected by altered intercostal and abdominal muscle contributions when trunk rotation postures, such as half and full rotation postures, are performed (Lee et al., 2010). The results of this study also revealed that the full rotation posture led to limited movement in the chest wall region but increased movement in the abdominal region, presumably due to reduced ribcage compliance, which altered diaphragm activity and increased contributions from the abdominal muscles (Cappello and De Troyer, 2004; Lee et al., 2010). Trunk rotation postures lower ribcage compliance and increase diaphragm activation (Cappello and De Troyer, 2004; Lee et al., 2010), but the overall lung volume is not expanded due to a lack of symmetrical muscle contraction. This study also verified that changes in diaphragm and sternocleidomastoid activity that were caused by rotation postures contributed to increasing the asymmetric ratio for diaphragm and sternocleidomastoid activation.
The results of this study should be interpreted in consideration of its limitations. The effects we investigated were short-term, thus, our study cannot confirm whether the effects will persist in the long term.
In summary, the findings of this study revealed that postural change during respiratory muscle training may affect chest wall movement. Particularly, because the rotation posture may limit chest wall movement and induce diaphragm asymmetry during training, we recommend clinical respiratory muscle training to be performed in neutral postures to improve postural alignment.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.