INTRODUCTION
Cerebrovascular disease, or stroke, is one of the leading causes of death and is the most common cause of adult disability with increasing prevalence in the aged population. Intracerebral hemorrhage (ICH) is a subtype of stroke and has a higher risk of morbidity and mortality than ischemic stroke or subarachnoid hemorrhage (Broderick et al., 1993). ICH occurs more frequently in the elderly than in the young, and age is the important factor affecting brain damage in ICH in both animals and human (Daverat et al., 1991; Gong et al., 2004; Lee et al., 2006). In a prospective study, Daverat et al. (1991) found that aged individuals experienced the higher mortality and less recover from ICH. Gong et al. (2004) showed that ICH caused more severe neurological deficits and slower recovery in the aged rats compared to those in the young rats.
Apoptosis, also known as a programmed cell death, is a form of cell death that constitutes part of a common mechanism in cell replacement, tissue remodeling, and the removal of damaged cells. Inappropriate or excessive apoptosis has been implicated in several types of neurodegenerative disorders including stroke (Johnson et al., 1995). Apoptotic mechanism contributes to the neuronal cell loss in the striatum following the collagenase-induced ICH in rats and humans (Gong et al., 2001; Lee et al., 2003b; Qureshi et al., 2003). DNA fragmentation after ICH represents apoptosis (Matsushita et al., 2000).
Physical exercise is known to ameliorate brain damage in stroke survivors (Ada et al., 2003) and reduce the incidence of stroke (Lee et al., 2003a). In animal studies, treadmill exercise reduced brain infarction volume and improved neurological deficits after ischemic stroke (Ang et al., 2003; Marin et al., 2003; Yang et al., 2003). Sim et al. (2005) showed that treadmill exercise suppressed the ischemia-induced neuronal cell death, and resulted in improvement of memory in gerbils. Previously, treadmill running decreased the lesion size through decreasing ICH-induced cell death in rats (Lee et al., 2003b).
The neuroprotective effect of exercise on stroke has been well documented. However, these results have relied on the use of young animals rather than aged animals despite the importance of ICH in the elderly. In the present study, we investigated the age-dependence of the effect of treadmill exercise on the ICH-induced neuronal cell death in the striatum using young and aged rats.
MATERIALS AND METHODS
Animals and treatments
Young (n=24, 8 weeks) and aged (n=24, 64 weeks) Sprague-Dawley male rats were used in this experiment. The rats in each age were further divided into three groups, respectively (n=8 in each group): the 8-week-old sham-operation group, the 8-week-old hemorrhage group, the 8-week-old hemorrhage with exercise group, the 64-week-old sham-operation group, the 64-week-old hemorrhage group, and the 64-week-old hemorrhage with exercise group.
Induction of hemorrhage
For the induction of hemorrhage, the rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p., Sigma Chemical Co., St. Louis, MO, USA) and placed in a stereotaxic frame. Through a hole drilled in the skull, a 26-gauge needle was implanted into the striatum at the following coordinates: 2.6 mm lateral to the midline, 0.7 mm anterior to the coronal suture, depth 4.5 mm deep from the surface of the brain. One μL of saline containing 0.2 U collagenase (Type 4; Sigma Chemical Co.) was infused over 1 min. The needle remained in the place for an additional 3 min after the infusion, and subsequently was withdrawn slowly.
Treadmill exercise
The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day. The exercise load consisted of running at the speed of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, with the 0° inclination. The rats in the exercise groups were scheduled to run on a treadmill from 24 h after ICH induction for 7 consecutive days. The animals in the non-exercise groups were left in the treadmill without running for the same period as the exercise groups.
Tissue preparation
The animals were sacrificed immediately after the completion of the last exercise. The animals were weighted and overdosed with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS) and subsequently fixed with the freshly prepared 100 mM phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde. The brains were removed and fixed in the same fixative overnight and transferred into 30% sucrose solution for cryoprotection. Serial coronal sections of 40 μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany).
Determination of lesion volume by Nissl staining
To determine the lesion volume, Nissl staining was performed as the previously described method (Lee et al., 2003b). Digitalized photographs of the Nissl-stained sections were taken, and the lesions were quantified by using an Image-Pro®Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA). The hemorrhagic areas were summed from six to eight coronal slices at different levels. Volumes in mm3 were calculated by multiplying the section thickness to the measured areas (Wang et al., 2003).
Determination of DAN fragmentation by TUNEL staining
For the visualization of the DNA fragmentation, TUNEL staining was performed using In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany) as the previously described method (Ji et al., 2013; Kim et al., 2002). The, sections were fixed in ethanolacetic acid (2:1) and rinsed. Then the sections were incubated with proteinase K (100 μg/mL), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.02% 3,3′-diaminobenzidine (DAB). The sections were mounted onto a gelatin-coated slide. The slide was air dried overnight at room temperature, and cover-slips were mounted using Permount®.
A single axial section through the center of the hemorrhagic lesion was analyzed. The number of TUNEL-positive cells was counted on a computer screen grid from four fields (250×250 μm in each field) within the region adjacent to the hemorrhagic core as the previously described method (Lee et al., 2003b).
RESULTS
Effect of treadmill exercise on the intrastriatal lesion volume
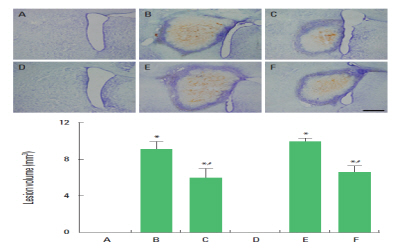
Fig. 1 shows the collagenase-induced lesion area in the striatum. The volume of lesion area was 9.10±0.83 mm3 in the 8-week-old hemorrhage group and 9.97±0.32 mm3 in the 64-week-old hemorrhage group, while the lesion was not detected in the shame-operation (control) groups. The volume of lesion area was 6.00±1.02 mm3 in the 8-week-old hemorrhage with exercise group and 6.53±0.74 mm3 in the 64-week-old hemorrhage with exercise group.
The effect of treadmill exercise on the number of TUNEL-positive cells
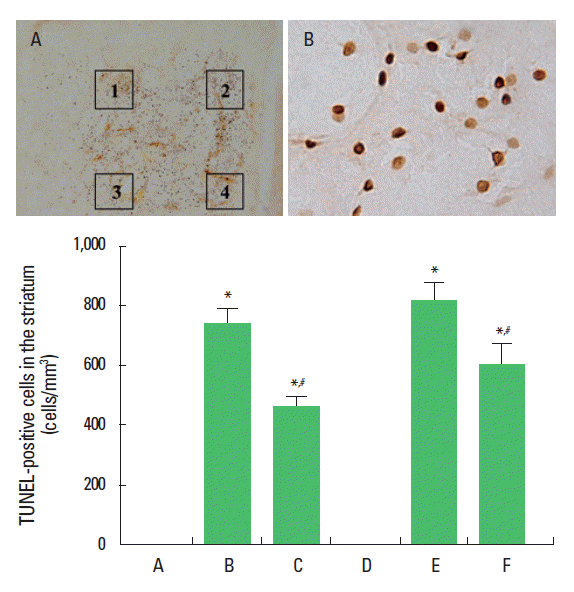
Fig. 2 shows the TUNEL-positive cells. TUNEL-positive cells were detected in the center and the peripheral area of the hemorrhagic lesion. In the sham-operation (control) groups, TUNEL-positive cells were barely detected. The number of TUNEL-positive cells was 744.02±50.91/mm2 in the 8-week-old hemorrhage group and 820.70±61.64/mm2 in the 64-week-old hemorrhage group. The number of TUNEL-positive cells was 464.42±32.43/mm2 in the 8-week-old hemorrhage group and 606.27±70.22/mm2 in the 64-week-old hemorrhage group.
DISCUSSION
In the present study, intrastriatal injection of collagenase into the striatum induced similar lesion size and there was no significant difference in the number of TUNEL-positive cells between young (8 week) and old (64 week) rats. Although the age-related increment in neurological deficits was reported in ICH rats (Gong et al., 2004), there has been no report on the effect of age on the ICH-induced lesion size. In humans, there was no difference in the infarct size with age after ischemic stroke (Engelter et al., 2003).
Brain edema formation after ICH is an important component of brain injury after ICH, causing neuronal cell death and long-term neurological deficits (Chu et al., 2004; Song et al., 2003). Brain swelling was more severe in the aged animals compared with young animals after ICH (Gong et al., 2004). Lee et al. (2006a) reported that higher level of inducible nitric oxide (iNOS) was observed in the senescence-accelerated prone mice compared to the senescence-resistant mice following ICH. iNOS is known to induce neuronal cell death following ischemic and hemorrhagic insults (Hanggi and Steiger, 2006). However, ischemic injury in the striatum, the levels of both excitatory and inhibitory neurotransmitters were not different with age (Shimada et al., 1989).
Our data show that treadmill running decreased the hemorrha ge-induced lesion size and inhibited the number of TUNEL-positive cells in both young and old rats. These results suggest that exercise induced neuroprotective effect against hemorrhagic stroke regardless of age. TUNEL staining has been used to identify cells with DNA fragmentation. Cells with DNA fragmentation are considered to be under the apoptotic cell death after ICH (Qureshi et al., 2003). The reduction of the lesion volume by the treadmill exercise might be ascribed to the suppression of apoptosis by tread-mill exercise.
It was also reported that treadmill exercise did not improve functional recovery and did not decrease lesion size in ICH rats (Auriat et al., 2006). The discrepancy compared with our finding may be explained by difference of the onset time of exercise after stroke, which is known as the important determinant for the efficacy of exercise intervention. In the present study, starting time of treadmill exercise was 24 h after ICH, whereas Auriat et al. (2006) started treadmill exercise 2 week after ICH induction. Early training after brain injuries promoted functional recovery without increasing tissue loss (Johansson and Ohlsson, 1996; Ohlsson and Johansson, 1995). Yang et al. (2003) showed that treadmill exercise initiated 24 h after ischemia reduced the infarct volume and promoted functional recovery, whereas the same intervention started one week later did not exert such effects. Similarly, previous study showed that lesion volume was decreased by exercise when started 24 h after ICH, but lesion volume was not decreased when started 72 h after ICH (Lee et al., 2005).
Here in this study, we have shown that treadmill exercise reduced the lesion volume and decreased the apoptotic neuronal death in the striatum of young and old rats following ICH. Although there was no age-dependent difference in the efficacy of treadmill exercise, treadmill exercise may provide therapeutic value against ICH by suppressing neuronal apoptosis regardless of age.