INTRODUCTION
Alzheimer’s disease (AD) is characterized by a complex of neuropathological, biochemical, and behavioral symptoms. AD gradually impairs learning ability and memory function, and the incidence of AD is 50–80% of dementia cases (Hosseini et al., 2013). Those with Alzheimer’s live on average 8 yr after their symptoms become noticeable to others, but survival rate ranges from 4 to 20 yr, depending on age and other health conditions (Hosseini et al., 2013).
The pathogenesis of AD and the cause are not known. However, in pathologic hypothesis for AD, there has been growing interest on the insulin signaling, and insulin resistance is involved in the pathogenesis of cognitive deficits in the neurodegenerative diseases (Hoyer, 2004; de la Nonte and Wands, 2005; Salkovic-Petrisic and Hoyer, 2007). The insulin-resistant brain state is thought to play a pivotal role in the pathogenesis of neurodegenerative disorders including AD (Lopez-Lopez et al., 2007). In particular, alteration of energy metabolism by enhancing of insulin resistance changes the memory function with decrement of neuronal growth factors in the brain (Dietrich et al., 2008; Freiherr et al., 2013).
Hippocampus is the important brain area in learning ability and memory function (Eichenbaum, 2004). In addition, hippocampus is one of the brain areas that cell proliferation continues throughout life in the adult mammals including humans (Eriksson et al., 1998; Lee et al., 2013). Alterations of hippocampal structures are implicated in the early symptoms of the AD (Braak et al., 2006), and neurons in the hippocampus are known to be vulnerable to the AD (Alkam et al., 2007).
Brain-derived neurotrophic factor (BDNF) is a small dimeric protein, and BDNF acts through high affinity binding with its receptor, tyrosin kinase B (TrkB). BDNF modulates neuronal growth and survival, and BDNF is implicated in learning and memory processes; therefore, dysfunction in BDNF is accompanied by cognitive deficits. Especially, BDNF is involved in the AD-related decline of neurogenesis, and the level of nerve growth factor is also decreased with AD (Chadwick et al., 2011; Hubka, 2006). This was also supported by the observation that administration of neurotrophic factors successfully improved the AD-related decrease in hippocampal neurogenesis (Hubka, 2006),
The beneficial effects of physical exercise on brain function and brain plasticity have been observed in numerous studies. Physical exercise has been recommended as a both preventive and therapeutic regimen in the management of patients with AD (Archer et al., 2011; Kim et al., 2014). However, the mechanisms of physical exercise on spatial learning ability under the AD conditions are not fully clarified.
We investigated the effects of treadmill exercise on spatial learning memory ability in relation with cell proliferation in the hippocampus using streptozotocin (STZ)-induced AD rats. For this study, radial 8-arm maze test, immunohistochemistry for 5-bromo-2’-deoxyuridine (BrdU), and western blot for BDNF and TrkB were performed.
METHERIALS AND METHODS
Animals and treatments
Adult male Sprague-Dawley rats, weighing 430± 20 g (40 weeks old), were used for the experiments. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The rats were housed under controlled temperature (23±2°C) and lighting (08:00 to 20:00 h) conditions with food and water available ad libitum. The animals were randomly divided into the following four groups (n=10 in each group): sham-operation group, sham-operation and treadmill exercise group, AD-induced group, and AD-induced and tread-mill exercise group.
All rats received 50 mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally 30 min before the starting of treadmill exercise, once for 3 days during first week.
Induction of AD
Animal model of AD was made using the previously described method (Jee et al., 2008). The rats were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France) and placed in the stereotaxic frame. Burr holes were drilled in the skull on both sides over the lateral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagital suture, and 3.6 mm beneath the surface of brain. Through a hole drilled in the skull, a 26-gauge needle was lowered manually into each lateral ventricle. The lesioned groups received bilateral intracerebroventricular (ICV) injection of STZ (1.5 mg/kg, 5 μL in saline). The rats in the sham-operation group underwent the same surgical procedures, but same volume of saline was injected instead of STZ. After surgery, the rats were housed individually and had access to food and water freely.
Exercise protocol
The rats in the exercise groups were forced to run on a motorized treadmill. The exercise load consisted of running at speeds of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, at an inclination of 0°. The rats in the exercise groups were scheduled to start treadmill running from 3 days after the ICV injection of STZ, and continued 28 days.
Radial 8-arm maze test
Spatial learning ability was determined using a radial 8-arm maze apparatus, according to the previously described method (Seo et al., 2013). The radial-arm maze apparatus consisted of a central octagonal plate (30 cm in diameter) and 8 radiating arms (50 cm in length and 10 cm in width). The apparatus was placed 1 m above the floor. A small receptacle filled with water (3 cm in diameter and 1 cm in depth) was located at the end of the arms. The rats were trained three times before the radial 8-arm maze test. The rats were deprived of water for 24 h, and were allowed to explore the water during test. The test was conducted on the 28 days after starting the treadmill exercise. The time spent in seeking water at the end of each arm was counted. The test was terminated when a rat found water in all 8 arms or when>8 min elapsed. Re-entry into the previously visited arms was counted as an error. In addition, the number of correct choice before the first error was counted.
Tissue preparation
The animals were sacrificed immediately after determining the spatial learning ability in the radial 8-arm maze test. The rats were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). Brains were dissected, and storage overnight same fixative, then it was transferred to 30% sucrose for cryoprotection. For the immunohistochemistry, the slices were coronal sectioned at 40 μm thick using a cryostat (Leica, Nussloch, Germany).
Immunohistochemistry for BrdU
To detect newly generated cells in the dentate gyrus, BrdU-specific immunohistochemistry was performed, according to the previously described method (Kim et al., 2010; Lee et al., 2013). Ten slice sections on average in the dentate gyrus were collected from each rat. The sections of 2.5 mm to 2.7 mm posterior from the bregma were used for immunohistochemistry. The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2 x standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 h with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Then, the sections were incubated for another 1 h with avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.03% diaminobenzidine (DAB), 40 mg/ml nickel chloride, and 0.03% hydrogen peroxide for 5 min. After BrdU-specific staining, determination of the differentiation of BrdU-positive cells was performed on same section using a mouse anti-neuronal nucleic (NeuN) antibody (1:1,000; Chemicon International, Temecula, CA, USA). The sections were washed three times with PBS, incubated for l h with a biotinylated anti-mouse secondary antibody, and processed with VECTASTAIN® ABC Kit. For staining, the sections were incubated in a reaction mixture consisting of 0.03% DAB and 0.03% hydrogen peroxide for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Western blot for BDNF and TrkB
Expressions of BDNF and TrkB were assessed by western blot analysis, according to the previously described method (Kim et al., 2010; Park et al., 2014). The hippocampal tissues were dissected and collected, and then were immediately frozen at −70°C. The right hemisphere were homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Hercules, CA, USA). Protein samples (30 μg) were separated on sodium dodecyl sulfate-polyacryl-amide gel and transferred onto a nitrocellulose membrane. The membranes were incubated with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 and then incubated overnight at 4°C with the following primary antibodies: mouse β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit BDNF antibody (1:500; Santa Cruz Biotechnology), rabbit TrkB antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Subsequently, membranes were incubated for 1 h with attempt secondary antibodies (1:2,000; Vector Laboratories), and ban detection was performed using the enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology).
Data analysis
The number of BrdU-positive cells in the hippocampal dentate gyrus was counted hemilaterally under a light microscope (Olympus, Tokyo, Japan). The area of the granular layer of the dentate gyrus from each slice was measured by by Image-Pro® Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). The number of BrdU-positive cells was expressed as the number of cells per mm2 of granular area in the dentate gyrus. For the confirmation of the expressions of BDNF and TrkB, the detected bands were calculated densitometrically using Molecular Analyst™, version 1.4.1 (Bio-Rad). Statistical analysis was performed using one-way ANOVA followed by Duncan’s post-hoc test, and the results are expressed as the mean±standard error of the mean (SEM). Significance was set as P<0.05.
RESULTS
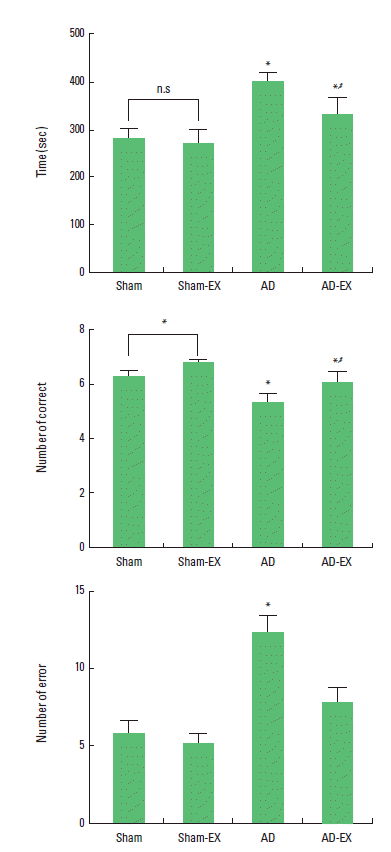
Effect of treadmill exercise on the spatial learning ability in radial 8-arm maze test
The time of successful performance, the number of the correct, and the number of error choice in the radial 8-arm maze test are presented in Fig. 1. The time of the successful performance was 281.25±18.20 sec in the sham-operation group, 268.50±30.03 sec in the sham-operation and treadmill exercise group, 399.70±17.98 sec in the AD-induced group, and 330.10±34.97 sec in the AD-induced and treadmill exercise group. The number of correct choice before the first error was 6.25±0.25 in the sham-operation group, 6.75±0.11 in the sham-operation and treadmill exercise group, 5.30±0.32 in the AD-induced group, and 6.00±0.44 in the AD-induced and treadmill exercise group. The number of error made before eight successful performance was 5.75±0.90 in the sham-operation group, 5.12±0.65 in the sham-operation and treadmill exercise group, 12.20±1.08 in the AD-induced group, and 7.70±0.95 in the AD-induced and treadmill exercise group.
These results show that AD-induced rats showed lower number of correct choice, increased completed time, and higher number of error than those in the sham-operation group. In contrast, tread-mill exercise reduced the number of error and completed time, and increased the correct choice in the AD-induced rats.
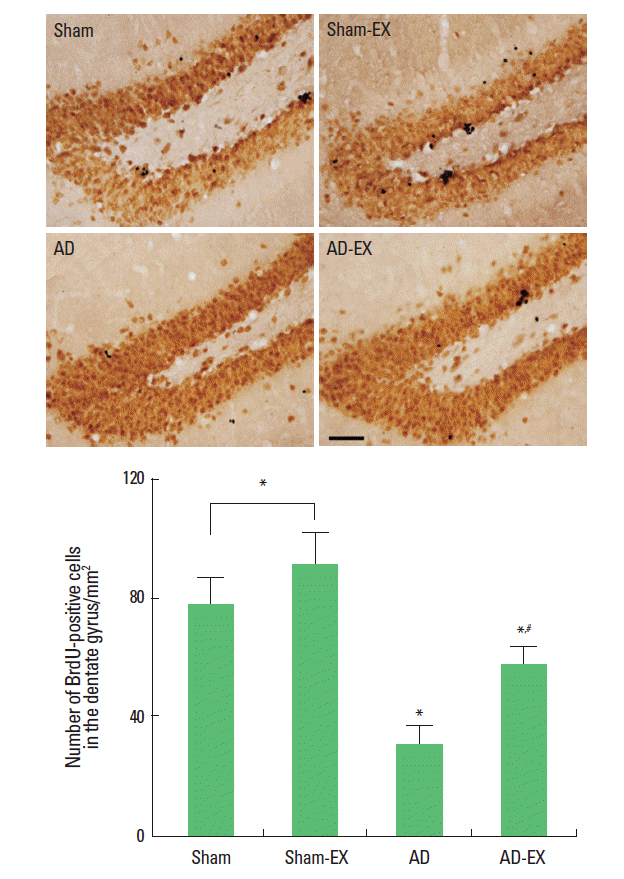
Effect of treadmill exercise on cell proliferation in the hippocampal dentate gyrus
Photomicrographs of BrdU-positive cells in the hippocampal dentate gyrus are presented in Fig. 2. The number of BrdU-positive cells was 77.61 ±8.58/mm2 in the sham-operation group, 90.79±10.62/mm2 in the sham-operation and treadmill exercise group, 30.22 ±6.15/mm2 in the AD-induced group, and 56.55±6.23/mm2 in the AD-induced and treadmill exercise group.
These results show that induction of AD reduced cell proliferation in the hippocampal dentate gyrus whereas treadmill exercise enhanced cell proliferation in the hippocampal dentate gyrus of the AD-induced rats.
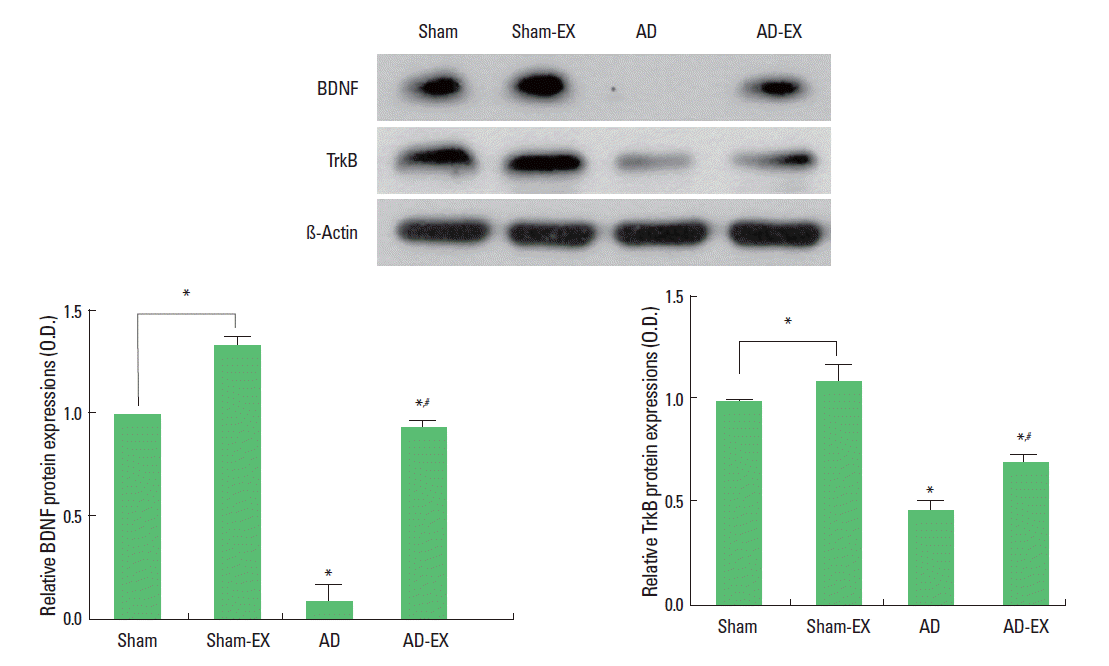
Effect of treadmill exercise on the expressions of BDNF and TrkB and in the hippocampus
When the level of BDNF (15 kDa) in the sham-operation group was set at 1.00, the level of BDNF was 1.33±0.04 in the sham-operation and treadmill exercise group, 0.09±0.07 in the AD-induced group, and 0.93±0.02 in the AD-induced and treadmill exercise group. When the level of TrkB (95–145 kDa) in the sham-operation group was set at 1.00, the level of TrkB was 1.09±0.08 in the sham-operation and treadmill exercise group, 0.46±0.04 in the AD-induced group, and 0.69±0.04 in the AD-induced and treadmill exercise group (Fig. 3).
These results show that induction of AD reduced BDNF and TrkB expressions in the hippocampus, whereas, treadmill exercise enhanced BDNF and TrkB expressions in the hippocampus of the AD-induced rats.
DISCUSSION
STZ at high dosage is commonly used to induce experimental diabetes mellitus in rats. However, ICV administration of STZ at low dosage in rodents does not alter peripheral glucose levels, and it is considered as the experimental model for late-onset sporadic AD (Muller et al., 2012; Sharma and Gupta, 2001). STZ-induced AD is associated with the impairment of learning ability and memory performance (Agrawal et al., 2011; Muller et al., 2012).
In the present study, time for the successful performance with the number of error choice was higher and the number of the correct was lower in the AD-induced rats than those in the sham-operation rat. These results reveal that induction of AD incapacitated spatial learning ability.
Alterations of energy metabolism by deficits in brain insulin levels have been consistently linked with the dysfunctions of neurotropic factors and neuroplasticity in the experimental animal models and AD patients (Craft, 2006). In particular, during dys-function of glucose consumption in the AD, BDNF was down-regulated (Hubka, 2006). When the immune system is activated, the secretion of neurotrophic factors is inhibited, and these events might be the underlying mechanism of the AD-induced dysfunctions of memory and plasticity (Chadwick et al., 2011; Hubka, 2006). BDNF is an important intercellular signaling molecule mediating neurogenesis, synaptic plasticity, and cell survival, and it plays a crucial role in learning and memory processes (Lee and Son, 2009). Suppression of BDNF by AD decreased neurogenesis with reduced memory function (Kim et al., 2014). Impairment of neurogenesis in the hippocampus is closely implicated in the deterioration of learning ability and memory function (Heo et al., 2014; Kim et al., 2010). ICV administration of STZ decreased expressions of neurotrophic factors including BDNF and also reduced neurogenesis in the hippocampus (Arabpoor et al., 2012; Shonesy et al., 2012).
In the present study, induction of AD decreased the expressions of BDNF and TrkB in the hippocampus. Cell proliferation in the hippocampal dentate gyrus was also suppressed in the AD-induced rats. These results indicate that induction of AD reduced BDNF and TrkB expressions in the hippocampus, and resulted in the suppression of cell proliferation in the hippocampal dentate gyrus.
Physical exercise is known to alleviate memory deficits by aging and neurodegenerative diseases (Kim et al., 2013; Sim et al., 2005). Treadmill running and wheel running are well documented to increase new cell formation and survival in the rodent hippocampus (Kim et al., 2013; van Praag et al., 1999). BDNF is an important factor for exercise-induced cell proliferation in the hippocampus (Gómez-Pinilla et al., 1998; Kim et al., 2013). Clinical evidences have shown that exercise decrease the risk for developing cognitive impairment and dementia in AD (Archer, 2011).
In the present study, treadmill exercise enhanced BDNF and TrkB expressions in the hippocampus of rats with ICV injection of STZ, resulting in increment of cell proliferation in the hippocampal dentate gyrus.
Here in this study, we have shown that treadmill exercise alleviated AD-induced deficits of spatial learning ability through enhancing of cell proliferation in the hippocampus. The enhancing effect of treadmill exercise can be ascribed to the increasing effect of treadmill exercise on BDNF expression. The present study suggests that treadmill exercise can be a useful strategy for treating memory impairment induced by several neurodegenerative diseases.