INTRODUCTION
High-intensity interval exercise is characterized by repeated bouts of high-intensity exercise interspersed with short rest periods or low-intensity exercise and forms the basis of training for team sports such as football. High-intensity interval training is frequently used in the development of athletic training programs because of its effectiveness in promoting numerous morphological and metabolic adaptations in skeletal muscle (Morton et al., 2009). Similarly, such forms of training have recently received much attention in the literature as a potent and time-efficient strategy for inducing numerous physiological adaptations that resemble traditional endurance training despite a low total exercise volume (Little et al., 2010). High-intensity interval training therefore represents an increasingly popular form of activity for enhancing both health and performance.
Athletes routinely undergo a temporary drop in the capacity to produce force following high-intensity exercise (Thorlund et al., 2009). Fatigue is defined as acute impairment of exercise performance that includes both an increase in the perceived effort necessary to generate a desired force and the eventual loss of the ability to produce that force after exercise (Davis and Bailey, 1997). A short-term drop in performance is, however, normally restored within 24 h. In contrast, muscle damage, which is characterized by muscle soreness and tenderness and a reduced capacity to generate force, frequently occurs following intense exercise, unfamiliar exercise, or eccentric contractions (Mathur et al., 2010). Symptoms generally occur within 24 h, peaking between 24 and 72 h, and lasting up to 5–7 days post-exercise (Cleak and Eston, 1992, Jones et al., 1986), and these symptoms are frequently referred to as delayed-onset muscle soreness.
It is important to characterize the physiological responses to different forms of high-intensity intermittent exercise in specific populations so that appropriate experimental designs can be established in future studies. Exercise-induced muscle damage leads to increased phagocytic cell competition, which affects cell signaling in many biological systems (Hancock et al., 2001; Suzuki et al., 1999). The use of a non-damaging exercise protocol provides a more controlled methodological approach for examining the effects of exercise per se on molecular and cellular responses in human skeletal muscles (Morton et al., 2006). The aim of this study, therefore, was to determine the physiological responses to a high-intensity intermittent running protocol in moderately trained subjects accustomed to performing frequent high-intensity exercise. It was hypothesized that such a running protocol would lead to symptoms associated with fatigue rather than those consistent with delayed-onset muscle soreness.
MATERIALS AND METHODS
Subjects
Ten healthy, active men volunteered to participate in the study (mean±SD: age, 31±7.1 yr; height, 174±4 cm; body mass, 74±8 kg; and maximal oxygen uptake [VO2max], 58±7.1 mL·kg−1·min−1). After details and procedures of the study had been fully explained, all subjects gave written informed consent to participate. Subjects refrained from exercise and alcohol and caffeine intake for at least 48 h prior to the intermittent-exercise test session. Subjects had no history of neurological disease or musculoskeletal abnormality and were not under any pharmacological treatment during the course of the study. The study was approved by the Ethics Committee of Liverpool John Moores University.
Familiarization
Subjects underwent extensive familiarization prior to participating in the study. During such sessions, the subjects were introduced to and familiarized with the procedure of maximal voluntary contraction (MVC) of the quadriceps (4-s duration). Approximately 3–4 contractions were performed during each session. Familiarization sessions were repeated until the subjects’ MVC force demonstrated a plateau effect between subsequent sessions. This level of initial consistency was usually achieved within 3–5 sessions (mean±SD, 4±2 sessions), after which subjects were then familiarized with high-intensity intermittent exercise. Subjects were then considered eligible to participate in the study.
Procedures
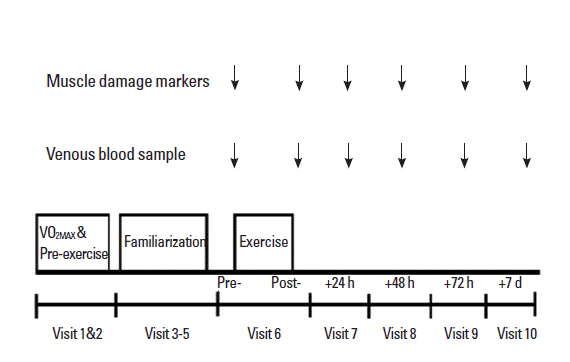
Prior to the completion of the experimental trials, all subjects completed pre-test determination of maximal oxygen uptake to facilitate calculation of the running speeds required for the intermittent-exercise protocol. Each subject was required to perform a high-intensity intermittent exercise running protocol on a motorized treadmill (HP Cosmos, Pulsar, Germany). MVC of the quadriceps muscle was determined immediately before and after exercise and at 24 h, 48 h, 72 h, and 7 days following exercise. At the same time, a venous blood sample was drawn for determining systemic indicators of muscle damage (CK and myoglobin). A schematic illustration of the experimental design is shown in Fig. 1.
On the day of the exercise trial, subjects arrived at the laboratory having completed appropriate diet and exercise diaries in order to monitor diet and physical activity levels. Subjects were instructed to ingest water 5 mL/kg body mass 2 h before arriving at the laboratory. Upon arrival at the laboratory nude body mass (kg; Seca, Birmingham, UK) was measured, and a heart rate monitor (Polar S610i, Kempele, Finland) was positioned across the chest. Following completion of the baseline assessments subjects commenced the intermittent-running protocol. Heart rate was continuously monitored and recorded at 5-s intervals during the protocol. A finger-prick blood sample for determining blood lactate concentrations (Lactate Pro, Arkray, Japan) and rating of perceived exertion (RPE) (Borg, 1970) were obtained immediately after each exercise bout. Venous blood samples were analyzed for CK activity and Mb concentration using a clinical chemistry analyzer (RX DaytonaTM, Randox, Crumlin, UK). The within-run precision of this device was assessed according to coefficient of variation (CVs; CK=0.7%, Mb=5.2%).
Exercise protocols
Each subject completed a standard incremental treadmill test on a motorized treadmill (HP Cosmos, Pulsar, Germany) for determination of maximal oxygen uptake. The protocol commenced at a treadmill speed of 10 km/h for 4 min followed by 2-min stages of 12 km/h, 14 km/h, and 16 km/h. Upon completion of the 16-km/h stage, the treadmill incline was increased by 2% every 2 min thereafter until volitional exhaustion. Maximal oxygen up-take was taken as the highest oxygen uptake value attained in any 10-s period according to the following endpoint criteria: (1) heart rate within 10 beats/min of age-predicted maximum, (2) respiratory exchange ratio (RER)>1.1, and (3) plateau of oxygen consumption despite increasing workload (Gilman, 1996). Expired fractions of oxygen and carbon dioxide were analyzed via an online gas analysis system (Oxycon Pro, Jaeger, Wuerzberg, Germany).
Subjects performed 60 min of high-intensity intermittent exercise on a motorized treadmill (HP Cosmos, Pulsar, Germany). The intermittent-running protocol consisted of a 10-min warm up at a running velocity corresponding to 70% of maximal oxygen up-take. This was followed by eight 3-min bouts at a running velocity corresponding to 90% of maximal oxygen uptake interspersed with 3-min active recovery periods (1.5 min at a velocity corresponding to 25% of maximal oxygen uptake followed by 1.5 min at velocity corresponding to 50% of maximal oxygen uptake). A 5-min cool-down period (50% of maximal oxygen uptake) was undertaken following completion of the final exercise bout.
Maximal isometric quadriceps force
The isometric force of the quadriceps of the subject’s dominant leg was measured with the subjects in an upright sitting position with the trunk vertical and 90° flexion in the hip and knee. Extraneous movement of the upper body was limited by 2 cross-over shoulder harnesses and an abdominal belt. Quadriceps muscle force was measured from the ankle, where the attachment was connected to a strain gauge by a metal force transducer (previously calibrated with known weights). Subjects completed 5 trials of 100% MVC (4-s duration) following a standard warm-up period. A 3-min rest period was provided between each trial in an attempt to eliminate the effects of fatigue (Newman et al., 2003). Subjects were given standardized strong verbal encouragement during each trial, and real-time visual feedback of their performance was provided via the projection of the computer display onto a large screen placed in front of the subject. MVC of the quadriceps muscle was determined in our laboratory according to Morton et al. (2005). CVs for each subject’s MVC over the 7-day period were 4.3%, as determined in a previous study.
Subjective estimation of muscle soreness
Ratings of perceived muscle soreness were assessed using a visual analog scale (VAS) (Zhang et al., 2000). The scale consisted of “no pain” at one end of a 100-mm line and “extremely sore” at the other end. Muscle tenderness was assessed via a push-pull hand-held dynamometer (Model FD130, Mecmesin, Italy). While subjects remained in a standing position, pressure was applied over the distal myotendinous junction (measured as 5 cm above the superior pole of the patella) (Sellwood et al., 2007) as well as the mid-belly of the rectus femoris muscle (midway between lateral condyle of the knee and head of the femur). These sites were placed along a datum line from the mid-patella to the lateral iliac crest (Baker et al., 1997) and marked so that the same site could be tested on each occasion. If subjects developed a hematoma because of the pressure applied during a previous measurement, subsequent measurements were taken just lateral to the datum line. All measurements were taken on each subject’s right side. Pressure was applied at an even rate of approximately 1 kg/s (Fischer, 1987) until the subject pointed out that the feeling had changed from “pressure” to “discomfort”. Measurements were taken 3 times at each site sequentially with a minimum of 1 min between tests. Measurements were recorded to the nearest 0.25 kg and the mean score was calculated (Cleary et al., 2005).
Statistical analysis
All data are presented as mean±SD. Any systematic changes in MVC, VAS, CK, Mb, blood lactate, and RPE during the intermittent-running protocol and for the subsequent 7-day period were assessed using one-way within-subjects general linear model (GLM). Post-hoc analysis using the Newman-Keuls test was undertaken to examine which time points were significantly different from baseline, with probability values of <0.05 assumed to indicate statistical significance.
RESULTS
Physiological responses to intermittent exercise
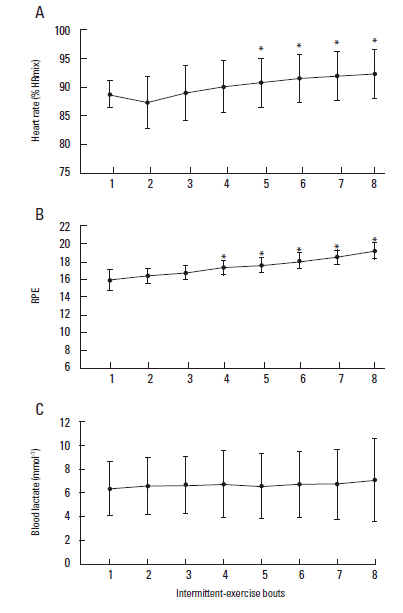
Mean heart rate significantly increased (Fig. 2A) during the intermittent protocol with values of 165±11 beats/min and 171±12 beats/min observed during the first and final bout, respectively (P<0.001). These values equated to 88.5±2.4% and 92±4.3% of recorded maximum HR. A similar increase in RPE was also observed, with values of 15.3±1.2 during bout 1 and 18.6±0.9 during the final, 8th bout (P<0.001; Fig. 2B). Blood lactate concentrations were significantly elevated following bout 1 compared with resting values (1.2±0.3 mmol/L vs 5.4±2.4 mmol/L; P=0.03). However, blood lactate concentration did not increase further during the remaining exercise bouts (P=0.42; Fig. 2C).
Indices of muscle damage
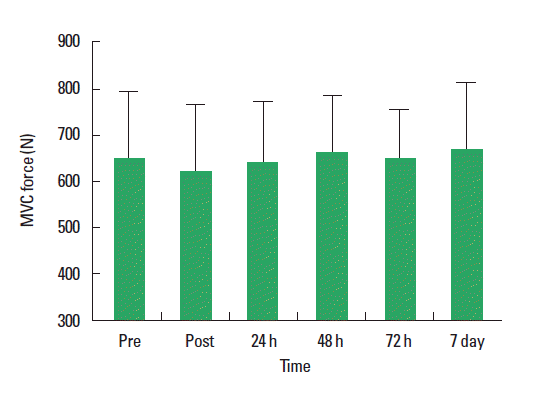
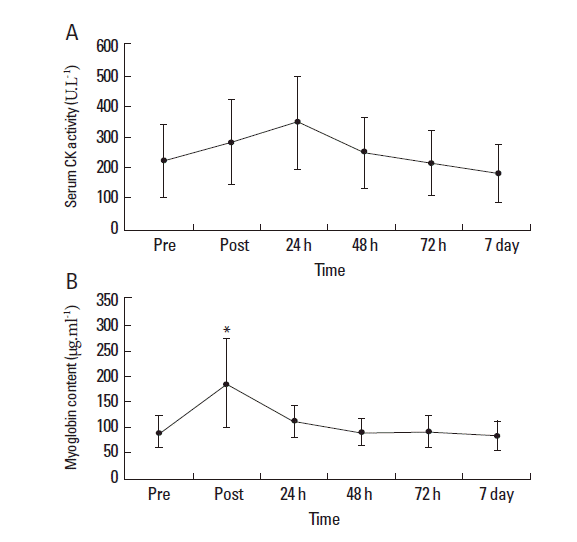
Mean baseline MVC score for the quadriceps was 648.3±148.5 N. No significant reduction in MVC was observed immediately following completion of the last exercise bout (623.9±143.6 N) or during the subsequent 7-d period compared to pre-exercise values (P=0.59) (Fig. 3). Ratings of perceived muscle soreness were increased at 24 h (P=0.081) and 48 h (P=0.017) post exercise compared to pre-exercise values (Table 1). However, ratings returned towards baseline values from 72 h onwards. Neither mid-belly nor musculotendinous muscle tenderness increased at any stage during the 7-day testing period. CK concentrations did not significantly increase following exercise (P=0.96; Fig. 4A). Mb content was significantly increased following intermittent exercise compared with pre-exercise rest (P=0.01; Fig. 4B). However, values returned to pre-exercise concentrations after 24 h.
DISCUSSION
The findings from the present study indicate that the high-intensity intermittent running protocol induced changes in physiological and subjective indices consistent with acute fatigue as opposed to those changes normally associated with exercise-induced muscle damage. This protocol can therefore be used in future studies to determine the influence of high-intensity exercise on physiological responses to molecular adaptation in moderately trained individuals.
High-intensity intermittent exercise has recently received much interest in the scientific literature. For example, recent work has shown that intermittent exercise is not only effective in enhancing aerobic and anaerobic performance in elite athletes (Dellal et al., 2009; Tanisho and Hirakawa, 2009) but also serves as an efficient and effective means of promoting increases in aerobic fitness in recreational populations relative to traditional forms of endurance exercise (de Souza et al., 2007). This particular model of high-intensity intermittent running has been used in our laboratory before the induction of oxidative adaptations in both gastrocnemius and vastus lateralis muscles (Morton et al., 2009). Furthermore, from a psychological perspective, high-intensity intermittent exercise is perceived to be more enjoyable than continuous exercise when matched for average intensity, duration, and total work done (Bartlett et al., 2011).
The present study shows that blood lactate values were significantly greater after the first bout of exercise (5.4±2.4 mmol/L) compared to baseline (1.2±0.3 mmol/L) and that the value gradually increased by the end of the exercise bout (7.3±3.8 mmol/L). Mean HR during the exercise bouts was more than 87% of HR-max and the HR continued to increase to 92% of HRmax at the end of the exercise bout. RPE score was 15 (hard) following the first bout of exercise and increased to 19 (extremely hard) following the final exercise bout. In relation to our physiological data, it is noteworthy that the exercise protocol in current study was more physiologically demanding than the protocol of running at 90% of maximal oxygen uptake for 6 repeated intervals, each of 3-min duration, which has been used in our laboratory (Bartlett et al., 2011; 2012).
Intense exercise causes muscle fatigue that can be recovered from within a few hours by using appropriate recovery strategies (Peiffer et al., 2010). However, following unfamiliar high-intensity exercise, especially eccentric exercise, disruption of internal structures characterized by myofibrillar disorientation and damage to the cytoskeletal framework may occur in the absence of any metabolic disturbance (Green, 1997). Decrements in muscle force patterns and their time course serve as a means by which to differentiate between the symptoms of muscle fatigue and muscle damage (Jones et al., 1986, Newham et al., 1983). To observe muscle damage induced by exercise, both directly, via measurements at the cellular level, and indirectly, via measurements of changes in various indices of muscle function, can be applied. One of the most appropriate and valid ways to quantify muscle damage following exercise is the impairment of the ability of skeletal muscle to produce force (Faulkner et al., 1993, Warren et al., 1999). Indeed, exercise-induced muscle damage leads to significant declines in MVC by 48 h post-exercise (Bailey et al., 2007). In the current study, MVC was not significantly reduced relative to baseline either immediately following exercise or at any time point during the subsequent 7 days. We observed a relatively small decrease both immediately after and 24 h post-exercise that returned to pre-exercise levels at 48 h. In addition, there were no significant reductions during 7 the days post-exercise. This failure to observe marked reductions in strength loss suggests that muscle damage typically characterized by long-lasting decreases in force-generating ability were not evident following the current high-intensity interval protocol in the moderately trained subjects (Brown et al., 1997, Eston et al., 1996, Murayama et al., 2000, Nosaka and Sakamoto, 2001).
Elevations in CK and Mb are frequently used as indicators of muscle damage, with increased concentrations frequently reported for up to 5 days after damaging exercise (Chen et al., 2007). In the present study, CK concentrations showed a tendency to increase post exercise and 24 h post exercise, with concentrations returning to pre-exercise levels after 48 h. Similarly, Mb concentrations significantly increased following exercise but returned to pre-exercise levels at 24 h post exercise. As a result, the changes in CK and Mb currently observed are representative of the symptoms of non-damaging nature of the exercise (Chen et al., 2007). The acute onset of perceived muscle soreness (i.e. increase in VAS) immediately after exercise and at 48 h are more related to the accumulation of byproducts that are induced either by metabolism or contraction (Miles and Clarkson, 1994) rather than delayed-onset muscle soreness, which is more commonly associated with muscle damage (Cheung et al., 2003). This is supported by the failure to observe any significant changes in muscle tenderness (mid-belly, musculotendinous) during the recovery period.
In summary, our results show that the current protocol does not lead to reductions in MVC or marked alterations in CK, Mb, or muscle tenderness. These data suggest that the current protocol does not lead to physiological changes consistent with the effects of damaging exercise. By separating exercise-induced molecular changes at the cellular level in response to exercise per se from immune responses caused by exercise-induced damage, a more controlled methodological approach to examining the effect of exercise on muscle adaptations can be achieved.