INTRODUCTION
Thanks to exercise, any bodily activity performed to make the muscles powerful, it is possible to decrease the level of surgical risks and to create a stronger immune system (Hu et al., 2001; Stampfer et al., 2000). The research in the field revealed that exercise mainly strengthens the skeletal muscles (Pedersen, 2013). Matrix metalloproteinases (MMPs) are a family of Zn++ and Ca++ dependent neutral endopeptidases that degrade components of extracellular matrix (ECM). Exercise-induced injury in skeletal muscle leads to increased expression of MMPs (Carmeli et al., 2005). MMPs are of critical importance in the homeostasis of the ECM in skeletal muscle (Carmeli et al., 2004). Thanks to the ECM surrounding muscle fibers, structural support and protection is enabled and functional integrity of the fibers is maintained (Birkedal-Hansen, 1995). There are some factors that inhibit MMPs. The biological activities of MMPs are antagonized by tissue inhibitor matrix metalloproteinases (TIMPs), such as TIMP-1 (Johnston et al., 2008). MMPs are suppressed by TIMPs with the quality of inhibiting MMPs by binding to their active sites (Jugdutt, 2003; Tsuruda et al., 2004). HA is a high-molecular-weight polysaccharide found throughout the ECM (Chung et al., 2016). A number of several physiological functions and mechanisms are included in HA such as a barrier effect, water homeostasis, stabilizing the ECM (Lieb et al., 2000; Turino and Cantor, 2003). Physical exercise results in numerous alterations in the oxidant-antioxidant balance. There can be seen a number of benefits from moderate exercise which is done regularly. Physical activity increases free radical production and the antioxidant utilization (Cooper et al., 2002; Lachance et al., 2001). There is a negative effect of exhaustive exercise on muscles by creating damage because of increased reactive oxygen species production in the skeletal muscle (Golden et al., 2002).
The aim of this study was investigate the levels of MMP-1, TIMP-1, hyaluronic acid (HA), total antioxidant status (TAS), and total oxidant status (TOS) following acute and chronic exercising in rats.
MATERIALS AND METHODS
Animals and experimental conditions
Twenty-six Wistar Albino 2-month-old male rats (200–250 g) were obtained from the Experimental Research Unit of Our University. They were reared under the supervision of a veterinarian, kept in well-ventilated noises environment and allowed free axes to food and water. They were maintained on a 12/12-hr light-dark cycle under controlled temperature. All protocols used in this study were approved by the Local Ethics Committee on animal research (in our study were used tissues of the animals in study supported with PAUHDEK-2012/035 number).
Experimental design
The animals were selected randomly and divided into three experimental groups: control (n=10), acute (n=7), chronic (n=9). The control group was not trained (sedentary). Acute exercise group; for 1 week on the treadmill, 3 days/wk, 10 min/day, 20 m/min was run. Chronic exercise group; on the treadmill, for 4 weeks, 7 days/wk, 60 min/day, 0.1 m/min was run.
Blood samples and measurements
When it comes to the end of the experimental period, all the animals were anesthetized with ketamin/xylazine HCl (75 mg/kg/10 mg/kg intraperitoneally). Blood samples were collected in heparinized tubes from the abdominal aorta of rats under anesthesia. Plasma samples were separated from cells by centrifugation at 3,000 rpm for 10 min. and were stored at −80°C until analysis. The plasma MMP-1, TIMP-1, HA concentrations were measured by an enzyme-linked immunosorbent assay (ELISA) method using an rat ELISA kit (Diagnostic Product Corp., Los Angeles, CA, USA) in a multiplate ELISA reader (das, Digital and Analog Systems, Vimercate, Italy). Rel-Assay Diagnostic kits use to analyze TAS and TOS level in ELISA microplate reader.
Statistical analysis
Data was analyzed by IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation and categorical variables as number and percentage. Kruskal–Wallis and Mann–Whitney U-test were used for statistical analyses. Relation between continuous variables was analyzed with Pearson correlation coefficient.
RESULTS
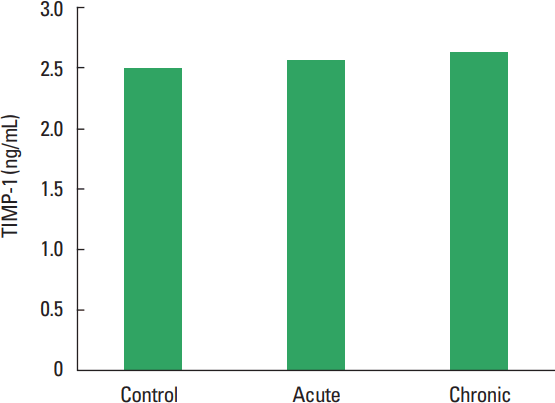
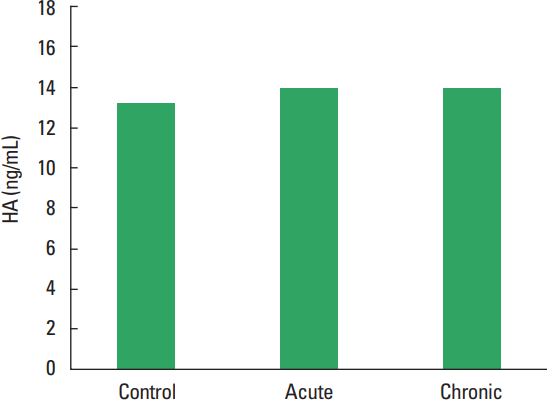
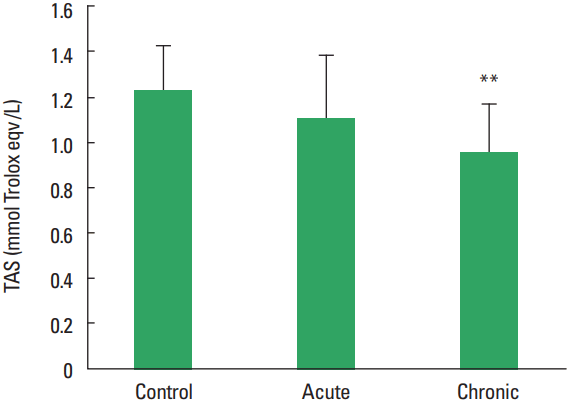
In current study, the MMP-1, TIMP-1, HA, and TOS levels not observed statistically significant difference among all groups (Figs. 1–Fig. 2Fig. 34). The levels of TAS and OSI (Figs. 5, 6) were significantly different between control and chronic exercise groups. However, no significant difference was seen the between the control and acute exercise groups in terms of the MMP-1, TIMP-1, HA, and TOS concentrations (P>0.05). A significant negative correlation between TIMP-1 and TAS was observed in all rats (r= −0.521, P=0.006). A negative correlation between TIMP-1 and TAS was observed in groups separately. But the negative correlation is not statistically significant. The plasma TOS was found to be significantly high in the chronic group compared to the control group (10.73±2.89 vs. 7.48±4.08 mmol H2O2 equivalent/L, P<0.05), whereas plasma TAS was found to be significantly lower in the chronic group than in the control group (0.95±0.21 vs. 1.23±0.20 mmol H2O2 equivalent/L, P<0.01) (Figs. 4, 5, respectively). Similar to the plasma TOS values, the OSI value was significantly higher in the chronic group than in the control group (1.14±0.28 AU vs. 0.64±0.39 AU, P<0.01) (Fig. 6).
DISCUSSION
According to our results, the MMP-1 level was not changed in acute and chronic exercise groups compared to sedentary control group. In a study by Urso et al. (2009), it was reported that the authors characterized the MMP response in human skeletal muscle after acute exercise, and MMP-1 and MMP-3 concentrations increased following 8 weeks of training (Urso et al., 2009). Using a rat model, Carmeli et al. (2005) have reported that the MMP-2 response to training depends on the intensity of exercise, such that high intensity training (~70% of maximal oxygen consumption) was needed to increase MMP-2 expression in the gastrocnemius muscle of rats, whereas training at ~50% of maximum was not (Carmeli et al., 2005). Certain stimuli, particularly those that induce high levels of mechanical stress such as eccentric (Koskinen et al., 2001; Heinemeier et al., 2007) or high-impact exercise (Carmeli et al., 2007) leads to the activation of the local production of MMPs in skeletal muscle. In a study, Rullman et al. (2007) reported that the MMP response in human skeletal muscle was investigated through biopsies taken immediately after a single bout of moderate intensity cycling exercise and it was observed after 120 min. There was an exponential increase in skeletal muscle mRNA and protein for MMP-9 immediately after the exercise until 120 min. Serum concentrations of MMPs are found to be the highest within a relatively short time period following a single bout of exercise (Rullman et al., 2007). Circulating MMP-2 protein is reported to increase in humans within 1 hr after exercise (Suhr et al., 2007). Some important derivations can be made through this data considering the MMP response to exercise in rat or humans. First, it is probable that the MMP response is transient since peak changes occurred either during or within the first few hours after an exercise bout. Second, the mechanism of induction might be related to the mode of exercise training or skeletal muscle (e.g., resistance-based training), depending on the nature and intensity of the exercise training (Urso et al., 2009). In our study, the fact that there is no increase in MMP-1 can be explained with the following two reasons; that is, MMP-1 increase was temporary and the level of exercise intensity is not sufficient.
In current study, any significant difference was not observed on TIMP-1 level between exercise and control groups. In the study of Hoier et al. (2012), mRNA levels of TIMP1 was found to be increased in response to acute exercise (after 2 weeks) but they were unaffected by training (after 4 weeks) (Hoier et al., 2012). In another study, thigh-force eccentric muscle contractions increased collagen remodeling and circulating levels of MMP and TIMP in humans and serum TIMP-1 was significantly elevated on days 1, 2, 3, 4, and 14 postexercise (Mackey et al., 2004). But Madden et al. (2011) showed that plasma levels of TIMP-1, inhibitor of the MMP-9, were also unchanged posteccentric exercise (Madden et al., 2011). In an acute exercise study, an increase in mRNA levels of TIMP-1 was observed as early as 6 hr after exercise. The levels peaked 1 or 2 days after running and attained control values 7 days after running in that study (Koskinen et al., 2001). This findings show that TIMP-1 activation is maintained during the early phase of muscle damage. TIMP-1, the enzyme that inhibits MMP activity seemed to be activated during the early phase of muscle damage. TIMP-1 is expressed in human skeletal muscle and that occurs shortly after exercise (Rullman et al., 2007). In our work is not sufficiently distinct difference between acute and chronic exercise time.
Plasma HA concentrations in our study did not discriminate among all groups. In a study by Hinghofer-Szalkay et al. (2002) six well-trained men performed incremental training until exhaustion (MAX), intensive (submaximal, SUB) and extensive exercise (moderate, MOD) on a bicycle ergometer. When the experimental group and the control group were compared, the plasma hyaluronan concentration (pHA) increased by 76% during 15-min MAX, by 44% during 30-min SUB and by 27% during 90-min MOD. After exercise (15 and 30 min), pHA decreased by 43% levels after MAX (P<0.05) and by 36% after SUB, respectively. In conclusion, reported that pHA steadily increased step by step during physical exertion, with a nonlinear increase of concentration/time slope with exercise intensity, the magnitude of the postexercise pHA decrease was proportional to the exercise-induced pHA increase, that made us think, elevated hyaluronan clearance with rising plasma levels after physical exertion (Hinghofer-Szalkay et al., 2002). Hyaluronan is an important safety factor of the edema and might quickly removed from the tissue (Lebel et al., 1988). The effects of exercise on plasma hyaluronan concentration have been shown in the literature. However, there is no clear dose response relations, particularly not those concerning in the decrease of pHA after muscular activity (Piehl-Aulin et al., 1991). In a study show that a significant decrease in hyaluronic acid plasma concentration during this strenuous exercise and authors speculate that decrease in hyaluronic acid concentration was due to an exercise-induced oxidative stress or exercise-induced shear stress enhancing hyaluronic acid incorporation into glycocalyx (Hrabarova et al., 2011).
In our present study, the plasma TOS was found to be significantly higher in the chronic group compared to the control group, whereas plasma TAS was found to be significantly lower in the chronic group than control group. Similar to the plasma TOS values, the OSI value was significantly higher in the chronic group than in the control group. When applied regularly, while moderate exercise has a beneficial effect on human health, intense exercise can produce damage in skeletal muscle and other tissues (McCutcheon et al., 1992). On the other hand, paradoxically exercise can induce oxidative damages. Huang et al. (2013) reported that physical exercise induced oxidative stress which could cause damage to muscle and liver tissues (Huang et al., 2013). Dalla Corte et al. (2013) demonstrated that elevation of oxidative damage markers in the brain, skeletal muscle, and blood after exhaustive exercise (Dalla Corte et al., 2013). The results of all these studies investigating effects of exercise in the literature demonstrated is conflicted. The study results are depending on the type, duration, intensity of the exercise as well as the age at onset of exercise. Maeda et al. (2001) indicated that chronic exercise might decrease oxidative stress probably by inhibiting some oxidative sources. In our work, the total plasma oxidant levels have increased while antioxidant levels decreased, although it is established in scientific literature that TAS levels increase with extended periods of exercise. Therefore, ıt is speculated that if the time allowed for the exercise had been longer, we may have seen a increase in the total antioxidant levels.
Our results have demonstrated that the MMP-1, TIMP-1, and HA release unchanged as a result of acute and chronic exercise training. Acute and chronic exercise does not have a positive effect on plasma MMP-1 and HA. Plasma TAS did was found to be significantly higher in the control group compared with that of the chronic group. It can say that on the oxidative response to exercise show that it varies with exercise intensity. Acute exercise did not have any adverse effect on the oxidant-antioxidant balance. In conclusion, our findings show that further research is needed to determine the functional significance of changes in the MMPs expression and total antioxidant levels in skeletal muscles in response to exercise training.