AbstractIn Korea, the first patient with a left ventricular assist device (LVAD) for destination therapy had successful implantation of a continuous-flow model in 2012. We investigated the safety and efficacy of exercise therapy with LVAD implantation 15 Korean patients. We retrospectively reviewed 15 patients (mean age, 67.4±11.6 years; 10 males, 5 female, left ventricular ejection fraction 23.6%±7.1%), including 4 with implanted continuous-flow and 11 an axial-flow LVAD. The New York Heart Association functional classification, ejection fraction, and quality of life were obtained. Survival rate, adverse events, admission rates, and enrollment rates in cardiac rehabilitation were investigated. Survival at 6 and 12 months was 100% and 89%, respectively. The New York Heart Association functional classification improved from 3.4±0.5 to 2.3±0.05 at 12 months postoperatively (P<0.0001). The ejection fraction significantly increased from 23.6%±7.2% on the preoperative day to 35.4%±14.2% at 1 year (P<0.0018). The quality of life was also improved at 1 year (P<0.0001). The most common adverse events were bleeding (56%) and dyspnea (44%). The number of admissions was 3.2 per patient-year. LVAD therapy is a safe and effective treatment option with exercise intervention for Korean patients waiting for heart transplantation or those who were ineligible for heart transplantation. A larger study with longer follow-up is needed to determine details clinical outcomes after LVAD.
INTRODUCTIONAccording to a 2017 report of the Ministry of Health and Welfare of Korea, mortality due to cardiac diseases including heart failure has progressively increased (Korean Statistical Information Service, 2017). The prevalence appeared to be increasing despite advances in medical therapy and cardiac surgery because of the increasing number of older adults with complex comorbidities (Komanduri et al., 2017; Roger, 2013).
Left ventricular assist devices (LVADs) have been shown to benefit these patients and can be used as a bridge to transplantation or destination therapy. Use of the LVAD as destination therapy has become more frequent in recent years, due to a donor organ shortage and an increasing number of older patients with terminal heart failure who are not eligible for heart transplantation. Several studies have demonstrated improved survival rates, exercise capacity and quality of life in patients with implanted LVADs (Jakovljevic et al., 2014; Loyaga-Rendon et al., 2015; Rogers et al., 2010; Slaughter et al., 2009). However, there is still a concern about adverse events such as infections, thromboembolic events, and mechanical failure (Forest et al., 2013; Genovese et al., 2009; Kirklin et al., 2008). Many studies have been reported by researchers in the United States or Europe because eligibility criteria for the procedure are very strict, and the procedure is not available in many countries (Kirklin et al., 2008). With an increase in heart failure patients, LVADs have become an option in the Asia-Pacific region. One study reported the clinical outcomes using LVADs (HeartMate II) in Singapore (Lim et al., 2014). Published in 2017, the registry for Mechanically Assisted Circulatory Support was the first study in Japanese patients (Nakatani et al., 2017). The clinical outcomes of successful 2nd generation LVAD implantation as destination therapy were reported in 2014 and the effect of exercise-based cardiac rehabilitation was also reported in Korean patients (Lee et al., 2014; Park et al., 2014).
Considering the increase in LVAD implantation in the growing population of heart failure patients, a descriptive study is needed to provide information on the clinical outcomes in Korean patients compared to those in other studies. Therefore, this study aimed to confirm the safety and efficacy of LVAD implantation in Korean patients and to provide descriptive data for clinical outcomes after LVAD implantation.
MATERIALS AND METHODSPatient selectionThe study population included 14 patients with heart failure due to dilated cardiomyopathy or ischemic cardiomyopathy and 1 patient with septic shock. These patients had not improved despite intensive medical and/or interventional treatments. In general, the criteria for LVAD implantation were based on the patient selection criteria from the REMATCH trial (Kirklin et al., 2008). Of 22 LVAD implantations as of July 2018 10 were used as a bridge to transplantation and 12 as destination therapy but only 15 patients had follow-up data available for analysis. Four patients received a Heartmate II (Thoratec Inc., Pleasanton, CA, USA) and 11 received a HeartWare (HeartWare Inc., Framingham, MA, USA). Eight patients underwent LVAD as a destination therapy and 7 as a bridge to transplantation (Fig. 1).
Exercise-based cardiac rehabilitationExercise therapy was started on postoperative day 3 in the intensive care unit (ICU). The first goal was to prevent pulmonary complications and to train inspiratory muscles by applying breathing exercises with a focus on incentive spirometry education. The patients were encouraged to try walking in the ICU and walking exercise was progressively increased on the ward. The exercise type and intensity progressed from bed exercise to stair use to enhance muscle strengthening. All patients participated in inpatient cardiac rehabilitation until discharge from hospital and were encouraged to enroll in outpatient cardiac rehabilitation. The cardiac rehabilitation participation was analyzed in this study.
Follow-upDuring hospitalization, adverse events such as death, stroke, severe right ventricular failure and LVAD-related infections (driveline exit site and pump pocket) were recorded. After discharge, patients were followed up for 12 months and adverse events after discharge, the unplanned readmission rate, median interval between discharge and first readmission, survival rate, length of stay in the ICU and hospital, New York Heart Association (NYHA) functional classification, ejection fraction, and quality of life were recorded for analysis of outcomes. The present study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2018-01-145-001), and the requirements for informed consents were waived due to the retrospective nature of this study.
Statistical analysisContinuous data are presented as mean±standard deviation or mean±standard error of the mean. Categorical data are presented as frequencies and percentages. NYHA functional classification, quality of life score, and ejection fraction were evaluated using the generalized estimating equation for repeated measure analysis. A P-value of <0.05 was considered statistically significant. All data were analyzed using the SAS software version 9.4 (SAS institute Inc., Cary, NC, USA).
RESULTSBaseline patient characteristicsPatient’ characteristics are summarized in Table 1. Mean age was 67.4±11.6 years. Four patients received HeartMate II and 11 received HeartWare devices. Dilated cardiomyopathy was the etiology of cardiac disease in 5 cases (33.3%), with ischemic cardiomyopathy in 5 (33.3%), systolic heart failure in 4 (26.7%), and septic shock with acute myocardial infarction in 1 (6.7%). Percutaneous coronary intervention had been performed in 41.2%, with coronary artery bypass grafting in 17.6%, and implantable cardioverter defibrillator implantation, permanent pacemaker placement, or aortic valve replacement in 11.8%. Cardiac resynchronization therapy had been performed in 5.9%. Other diagnoses included atrial fibrillation in 53.3%, hypertension in 33.3%, diabetes mellitus in 33.3%, and chronic kidney disease in 33.3%; risk factors associated with postoperative complications included stroke and chronic obstructive pulmonary disease in 20% (Table 1).
Adverse eventsAdverse events included cardiac arrhythmias (nonsustained ventricular tachycardia and atrial fibrillation), bleeding, hematoma in the driveline, abdominal or pericardial effusion, and pneumonia during hospitalization. Ventricular tachycardia (40%), and atrial fibrillation (6.7%), bleeding (40%), and effusion (33.3%) were the most common complications during hospitalization. Adverse events after discharge from the hospital included bleeding, stroke, hematoma, and effusion. The most common adverse event after discharge was gastrointestinal (46.7%) (Table 2).
Cardiac rehabilitation participation rateAll patients participated in inpatient cardiac rehabilitation after implantation according to our hospital protocol. Inpatient cardiac rehabilitation was conducted for an average of 64.5±82.1 sessions.
ReadmissionThe average length of stay in the ICU and hospital was 25.3±17.5 days and 103.7±51.7 days (range, 6–59 days), respectively. However, in analyzing length of stay for hospitalization, 2 patients were excluded because they remained in the hospital for 1 year. The median time until first readmission was 57.2 days. The readmission rate during the follow-up period was 77.0%, 83.3%, and 90.5% at 3, 6, and 12 months, respectively; 22 readmissions occurred in 13 patients (average: 1.7±1.3), 30 in 12 patients (2.2±2.1), and 38 in 10 patients (3.8±2.9) at 3, 6, 12 months from discharge, respectively. The most common reason for readmission was gastrointestinal bleeding (Table 3).
Clinical outcomesThe survival rate at 6 and 12 months was 100% and 89%, respectively. The mean preoperative NYHA functional classification was 3.4±0.5, with 1.9±0.3 on the postoperative day and 2.3±0.5 at postoperative 12 months. The NYHA functional classification was significantly improved on the postoperative day (P<0.0001) and at postoperative 12 months (P<0.0001). Cardiac function measured with ejection fraction showed significant improvement from 23.6%±7.2% on the preoperative day to 35.4%±14.2% at postoperative 1-year (P<0.0018) (Fig. 2). The physical and emotional component scores were significantly decreased from 35.1± 4.4 at baseline to 21.4±7.1 at postoperative l year (P<0.0001) and from 20.2±4.8 at baseline to 12.0±4.4 at postoperative l year (P<0.0001), respectively. The total score of quality of life was also significantly improved at postoperative 12 months (P<0.0001) (Fig. 3).
DISCUSSIONThis study was the first to report the long-term clinical outcomes of LVAD implantation in Korean patients. The important findings are survival rates of 100% and 89% at 6 and 12 months, respectively. Functional capacity measured using the NYHA classification and quality of life were improved after LVAD implantation. These results demonstrate that LVAD therapy is safe and effective for improving functional capacity and quality of life in Korean patients.
Patients with an implanted LVAD have shown greater improvement in cardiopulmonary function (Jakovljevic et al., 2014; Loyaga-Rendon et al., 2015). Exercise training in LVAD recipients can also improve exercise capacity (Mahfood Haddad et al., 2017). In the 3 months after implantation, the physical activity level increased in these patients, but thereafter remained unchanged until the 12-month follow-up (Jakovljevic et al., 2014). The exercise-based cardiac rehabilitation program was conducted in both the inpatient phase in this study. Inpatient cardiac rehabilitation was performed for an average of 64 days in all patients, but the participation rate in outpatient cardiac rehabilitation after discharge was very low. The 6-min walk test has been generally used to evaluate exercise capacity in patients with an implanted LVAD. In this study, we could not analyze improvement in exercise capacity because the test was not routinely performed during the follow-up period. However, functional capacity measured using the NYHA classification was improved after implantation of LVAD in this study. We also found improvement in cardiac function, with an increase in ejection fraction at 12 months. Authors thought that the improvement of these factors may be associated with participation in exercise-based cardiac rehabilitation. However, further randomized controlled trial is needed to identify the effect of exercise intervention in Korean patients with an implanted LVAD.
Survival rates at 6 and 12 months were 100% and 89%, respectively, in this study. One study (Haeck et al., 2015) reported a survival rate of 75% at 6 months, and the eighth INTERMACS report (all indications, n=15,000) showed that actuarial 1-year survival for continuous-flow LVADs was 80% (Kirklin et al., 2017). The survival rate in the present study was slightly higher than in a previous study (Haeck et al., 2015; Nakatani et al., 2017) and the INTERMACS report (Kirklin et al., 2017), but the small sample size should be considered in comparing survival rates. The most common cause of death was a neurological disorder, followed by multiple system organ failure, infection, device malfunction, and right heart failure (Kirklin et al., 2017). Another study reported that infection was the most common cause of death after LVAD implantation (Nakatani et al., 2017). In this study, two patients with LVAD implantation as a bridge to transplantation died due to acute heart failure and sarcoma at postoperative 252 and 315 days, respectively. Further study is needed with a larger sample size of Korean patients to analyze the differences between Korean data and other studies.
In the present study, the average length of stay in the ICU and hospital was 25 and 104 days, respectively. A study (Cotts et al., 2014) reported a median hospital length of stay of 20 days; risk factors for longer hospital stay included age over 64 years, history of coronary artery bypass graft, history of valve surgery, diabetes mellitus, ascites, and concomitant surgery. The present study showed a longer length of stay than in previous studies (Akhter et al., 2015; Cotts et al., 2014). The mean age in this study was 67.4 years, which could account for the longer hospital stay. Risk factors such as history of coronary artery bypass graft (17.6%), diabetes mellitus and hypertension (33.3%) were also present in our patients and may have affected length of stay. When all patients were divided into bridge to transplantation or destination therapy groups, the bridge to transplantation group (8 patients) had a longer hospital length of stay than the destination therapy group (7 patients). Understanding the differences among options to LVAD implantation may contribute to improved preoperative patient selection and management.
Longer hospital length of stay is associated with the occurrence of adverse events. The INTERMACS reports investigated adverse events after LVAD implantation (Kirklin et al., 2013). Early-onset adverse events after implantation included arrhythmias, tamponade, bleeding, and renal insufficiency, and late-onset adverse events included bleeding, right ventricular failure, infection, aortic valve pathology, thromboembolic events, and device failure (Genovese et al., 2009; Kirklin et al., 2008; Kirklin et al., 2013). Bleeding, cardiac arrhythmia, infection, and stroke were significant adverse events in postoperative year 1 (Kirklin et al., 2017). The most frequent event after LVAD implantation was gastrointestinal bleeding in previous studies (Kirklin et al., 2008; Uriel et al., 2010). We found that the primary adverse event was bleeding, followed by cardiac arrhythmia, and effusion during hospitalization. Bleeding was also the most common cause of adverse events after discharge in the present study. The types of adverse event were similar to those in the previous reports (Genovese et al., 2009; Kirklin et al., 2017; Uriel et al., 2010). Preoperative older age (>65 years) and ischemic etiology were significant risk factors for bleeding (Boyle et al., 2014). These factors may have been associated with the increased incidence of bleeding in our study. Patient selection for LVAD implantation should include screening for preoperative risk factors.
Readmission rate or frequency is an important clinical outcome, and several studies (Kimura et al., 2017; Vidula et al., 2018) have reported the average readmission frequency range of 1.34–1.79 per patient-year after discharge. The readmission frequency of 3.2 per patient-year in this study was slightly higher than that reported in previous studies. The mean time to first readmission was 57 days, which was longer than a previous report (Vidula et al., 2018) of 48 days but shorter than in a report by Kimura et al. (2017). The readmission frequency differed between implanted HeartMate-II or HeartWare devise (Haglund et al., 2015) but was not different between destination therapy and bridge to transplantation LVAD recipients (Bradner et al., 2013). Based on several studies (Bradner et al., 2013; Haglund et al., 2015; Joy et al., 2016), we considered gastrointestinal bleeding to be a risk factor for the increased readmission rate in this study, and this risk factor was associated with older age in patients enrolled in this study.
The quality of life has become an important parameter in evaluating clinical outcomes in LVAD recipients. Several studies (Haeck et al., 2015; Kato et al., 2015; Kirklin et al., 2017; Mahfood Haddad et al., 2017) demonstrated that quality of life after implantation was significantly improved in the physical and mental components. In our study, the quality of life measured at 12 months using the Minnesota Living with Heart Failure Questionnaire after implantation also showed improvement in both physical and mental components. Several factors, including right ventricular failure, female sex, and postoperative anxiety influenced quality of life after LVAD implantation (Kato et al., 2015).
This study had some limitations. First, the number of patients was small and we did not perform follow-up in all patients who received an implanted LVAD due to the 1-year limit. Second, comparison of clinical outcomes according to the type or purpose of LVAD implantation was not performed due to the small sample size. Third, this report was based on a single-center study and generalization of the results should be cautious.
In conclusion, this single-center study demonstrated that LVAD implantation in Korean patients with heart failure is an effective option to improve clinical outcomes. However, further studies with longer follow-up are needed to confirm the effect of exercise therapy in a larger group of Korean patients.
ACKNOWLEDGMENTSThe authors are grateful to professor of Kyunga Kim and researcher of Heejin Yoo (Statistics and Data Center, Research Institute for future medicine in Samsung Medical Center) for analyzing of data.
REFERENCESAkhter SA, Badami A, Murray M, Kohmoto T, Lozonschi L, Osaki S, Lushaj EB. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence, causes, and cost analysis. Ann Thorac Surg. 2015;100:884–889.
Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD; HeartMate II Clinical Investigators. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–888.
Bradner M, Whitson BA, Eckman P, Lacey A, Colvin-Adams M, Liao KK, John R. Hospital readmissions in left ventricular assist device (LVAD) recipients: analysis of bridge to transplant (BTT) and destination therapy (DT). J Heart Lung Transplant. 2013;32:4 Suppl. S99
Cotts WG, McGee EC Jr, Myers SL, Naftel DC, Young JB, Kirklin JK, Grady KL. Predictors of hospital length of stay after implantation of a left ventricular assist device: an analysis of the INTERMACS registry. J Heart Lung Transplant. 2014;33:682–688.
Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D’Alessandro D, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95:1276–1281.
Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Kay J, Siegenthaler MP, Bhama JK, Bermudez CA, Lockard KL, Winowich S, Kormos RL. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg. 2009;88:1162–1170.
Haeck ML, Beeres SL, Höke U, Palmen M, Couperus LE, Delgado V, Logeman EA, Maas JJ, Klautz RJ, Schalij MJ, Verwey HF. Left ventricular assist device for end-stage heart failure: results of the first LVAD destination program in the Netherlands. Neth Heart J. 2015;23:102–108.
Haglund NA, Davis ME, Tricarico NM, Keebler ME, Maltais S. Readmissions after continuous flow left ventricular assist device implantation: differences observed between two contemporary device types. ASAIO J. 2015;61:410–416.
Jakovljevic DG, McDiarmid A, Hallsworth K, Seferovic PM, Ninkovic VM, Parry G, Schueler S, Trenell MI, MacGowan GA. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol. 2014;114:88–93.
Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk factors and outcomes of gastrointestinal bleeding in left ventricular assist device recipients. Am J Cardiol. 2016;117:240–244.
Kato NP, Okada I, Imamura T, Kagami Y, Endo M, Nitta D, Fujino T, Muraoka H, Minatsuki S, Maki H, Inaba T, Kinoshita O, Nawata K, Hatano M, Yao A, Kyo S, Ono M, Jaarsma T, Kinugawa K. Quality of life and influential factors in patients implanted with a left ventricular assist device. Circ J. 2015;79:2186–2192.
Kimura M, Nawata K, Kinoshita O, Yamauchi H, Hoshino Y, Hatano M, Amiya E, Kashiwa K, Endo M, Kagami Y, Nemoto M, Ono M. Readmissions after continuous flow left ventricular assist device implantation. J Artif Organs. 2017;20:311–317.
Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156.
Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065–1072.
Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086.
Komanduri S, Jadhao Y, Guduru SS, Cheriyath P, Wert Y. Prevalence and risk factors of heart failure in the USA: NHANES 2013 – 2014 epidemiological follow-up study. J Community Hosp Intern Med Perspect. 2017;7:15–20.
Korean Statistical Information Service. Causes of death statistics in 2014 [Internet]. Daejeon (Korea): [cited 2017 Oct 4]. Available from: http://kostat.go.kr/portal/eng/pressReleases/1/index.board?bmode¼read&aSeq¼349053
.
Lee GY, Park SJ, Kim S, Choi N, Jeong DS, Jeon ES, Lee YT. The successful implantation of continuous-flow left ventricular assist device as a destination therapy in Korea: echocardiographic assessment. J Korean Med Sci. 2014;29:137–140.
Lim CP, Sivathasan C, Tan TE, Lim CH, Kerk KL, Sim DK. Use of left ventricular assist device (HeartMate II): a Singapore experience. Artif Organs. 2014;38:543–548.
Loyaga-Rendon RY, Plaisance EP, Arena R, Shah K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J Heart Lung Transplant. 2015;34:1005–1016.
Mahfood Haddad T, Saurav A, Smer A, Azzouz MS, Akinapelli A, Williams MA, Alla VM. Cardiac rehabilitation in patients with left ventricular assist device: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev. 2017;37:390–396.
Nakatani T, Sase K, Oshiyama H, Akiyama M, Horie M, Nawata K, Nishinaka T, Tanoue Y, Toda K, Tozawa M, Yamazaki S, Yanase M, Ohtsu H, Ishida M, Hiramatsu A, Ishii K, Kitamura S; J-MACS investigators. Japanese registry for mechanically assisted circulatory support: first report. J Heart Lung Transplant. 2017;36:1087–1096.
Park WH, Seo YG, Sung JD. Exercise therapy for an older patient with left ventricular assist device. Ann Rehabil Med. 2014;38:396–400.
Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS; HeartMate II Investigators. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834.
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251.
Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213.
Fig. 1Status of left ventricular assist device (LAVD) implantation. (A) Second generation: continue-flow LVAD device. (B) Third generation: HeartWare LVAD device. 
Fig. 2Clinical outcomes according to physical activity and cardiac function. (A) New York Heart Association functional classification. (B) Cardiac function with ejection fraction measured using echocardiography. 
Fig. 3The quality of life measured using the Minnesota Living with Heart Failure Questionnaire. (A) Physical component of quality of life. (B) Emotional component of quality of life. (C) Total score for quality of life. 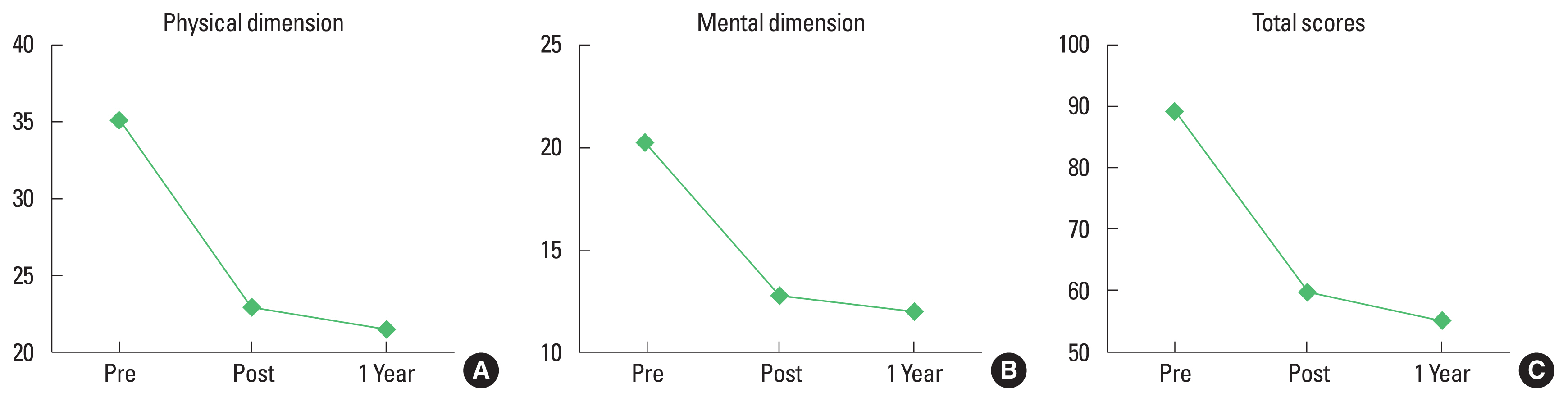
Table 1Baseline patient characteristics (n=15) Table 2Adverse events in the hospital and after discharge Table 3Clinical outcomes after implantation |
|
|||||||||||||||||||||||||||||||||||||||||||