INTRODUCTION
Chronic cerebral hypoperfusion (CCH), the main reason for vascular dementia (VaD), is caused by the thromboembolic events of the brain, results in progressive cognitive impairment (Iadecola, 2013). The cerebral cortex, hippocampus, and white matter are susceptible to hypoperfusion-induced lesions. A reduction of cerebral blood flow leads to hypoxia and oxidative stress. The hypoxia-induced oxidative stress causes mitochondrial dysfunction and neuronal cell death via the release of reactive oxygen species (Venkat et al., 2015). Although it is essential to understand the pathophysiology of the CCH for establishing the proper animal model to develop treatments, the pathology and mechanism of CCH have not yet been fully understood. Hence, it needs a new approach to understand the mechanism better.
The role of the cerebellum has recently been reestablished to represent a cognitive function. The description of the cerebellar cognitive affective syndrome (CCAS) and topographical study of the cerebellum indicate the cerebellar engagement into neural circuits relevant to cognition and emotion (Jacobs et al., 2018). CCAS signifies a deficit in the cognitive domain of executive function, spatial memory, and language caused by the cerebellar damage (Schmahmann and Sherman, 1998). The cerebellum is mainly related to cognitive function, especially related to spatial navigation, situated in the posterior lobe of the cerebellum. Damage to the posterior lobes of the cerebellum is associated with impairment of spatial working memory and knockout mice with cerebellar dysfunction have been shown to exhibit spatial navigation disorders (Tomlinson et al., 2014). Our previous study was confirmed that the loss of Purkinje cell that has contained plenty of calcium-binding proteins in the posterior lobe caused by CCH affects spatial navigation impairment (Lee et al., 2018).
It has been reported that the loss of Purkinje cells in the cerebellum is caused by glutamate release and excitotoxicity due to intracellular calcium imbalance (Welsh et al., 2002). Intracellular calcium homeostasis is vital in maintaining calcium-related signaling pathways and nerve function in healthy brains (Pchitskaya et al., 2018). Calcium channel, endoplasmic reticulum, calcium-binding protein, and mitochondria perform a buffer system to prevent calcium overload. Mitochondria are essential calcium modulators that absorb calcium to reduce cellular calcium levels. Mitochondria are regulating synaptic transmission, brain function, and cognitive function. Mitochondrial dysfunction has been found to be involved in the pathology of neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer disease, and Parkinson disease (Marambaud et al., 2009). Many diseases impaired neuroplasticity, which leads to cell dysfunction, or even cell death. Recently previous study, exercise strengthened mitochondria in the brain by increasing neuroplasticity and suppressing cell death (Seo et al., 2019).
Enhancing mitochondrial function may be a therapeutic approach to prevent the loss of Purkinje cells. It is a well-known intervention that treadmill exercise improves cognitive function by reducing the neuronal apoptosis and the responsiveness of astrocytes in the cerebellum following global ischemia and attenuates age-related Purkinje cell loss (Larsen et al., 2000; Seo et al., 2010). The study aimed to examine whether CCH induces a loss of Purkinje cells, and to investigate the effect of low-intensity treadmill exercise (LITE) on cognitive function by enhancing the mitochondrial calcium retention capacity in the cerebellum.
MATERIALS AND METHODS
Animals
We used adult male Wistar rats (body weight 80±10 g, 4 weeks old) in this experiment. We randomly divided the rats into three groups: the sham group (n=8), the bilateral common carotid arteries occlusion (BCCAO) group (n=8), and the BCCAO and treadmill exercise (BCCAO+Ex) group (n=8). All animals were inhabited under controlled conditions of temperature (25°C±2°C), humidity (50%–55%), and light (12-hr light-dark cycles), with access to food and water ad libitum. All animal experimental procedures were approved by the National Institutes of Health and the guidelines of the Korean Academy of Medical Science. This study obtained approval by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP [SE]-17-138).
Bilateral common carotid arteries occlusion
We anesthetized the rats in 3% halothane in fresh gas flow composed of an N2O:O2 (70:30). A ventral right incision in the neck was performed to expose the right common carotid artery, which was carefully separated to avoid damage of surrounding tissues particularly near a vagus nerve. The right common carotid artery was ligated doubly with 3-0 silk (Ailee, Seoul, Korea) below the carotid bifurcation. A left common carotid artery occlusion was performed in the same procedure at 1 week after the right common carotid artery surgery. Sham-operated animals underwent the same operation process without the vessel ligation.
LITE protocol
Before the surgery, the rats were engaged on a treadmill for 30 min for 8 weeks, according to the previously described method (Heo et al., 2014). The LITE procedure was made up running at 2 m/min for the first of 5 min, at 3 m/min for the next 5 min, and then at 5 m/min for the last 20 min at 0° of inclination. The rats in the nonexercise groups were put in the treadmill without running for the same period as the exercise group.
Radial arm maze test
The radial 8-arm maze test was performed to assess spatial working memory ability after 3 weeks of BCCAO. The radial 8-arm maze consists of eight equally spaced arms (60 cm×12 cm×12 cm) radiating from a central octagonal platform, placing the rat on the central octagonal platform and collecting water hidden at the tip of the arm. On the first day of training, rats can enter each arm arbitrarily to collect water hidden in each arm for 8 min. After 24 hr, the test day scores the arm selection when all four paws of the rat into the arm. On test day after 24 hr, rats will go into each arm to find water, and revisiting a previously visited arm will result in an error record. The number of correct selections before the first error was measured, and the error was counted when they revisited the arms.
Immunohistochemistry
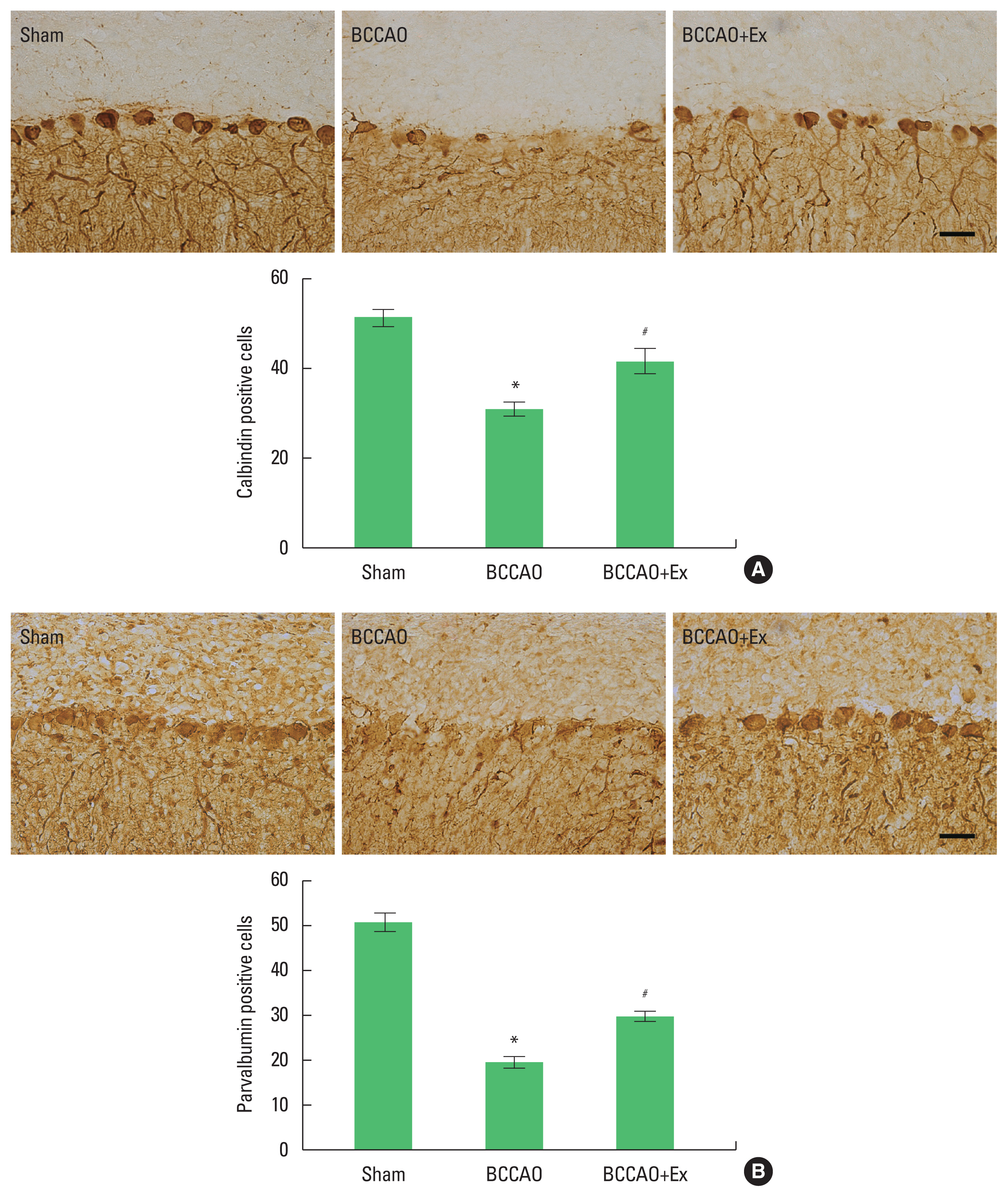
Serial sagittal sections of 40-μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany). Immunohistochemistry was performed to investigate the expression of calbindin D28k and parvalbumin. Free-floating sections were initially incubated in 3% H2O2 for 30 min at room temperature. After being blocked with 10% normal horse and rabbit serum for 1 hr, the sections were incubated overnight at 4°C with calbindin D28k antibody (1:500; Abcam, Cambridge, UK), and parvalbumin antibody (1:1,000; Abcam). The sections were then incubated for 2 hr with the biotinylated rat and mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 hr at room temperature. The bound secondary antibody was then amplified using a Vector Elite ABC kit (Vector Laboratories) for 1 hr at room temperature. For staining, the sections were incubated in a solution consisting of 0.02% diaminobenzidine and 0.03% H2O2 in 50 mM Tris-HCl (pH, 7.6) for approximately 5 min, following which they were washed with phosphate-buffered saline and mounted onto gelatin-coated slides. Cover slips were mounted using Permount (Fisher Scientific, Waltham, MA, USA). The number of positive cells per section was counted in 5 random fields from every specimen with a Nikon Eclipse 80i microscope (magnification, ×40; Nikon Corporation, Tokyo, Japan).
Mitochondrial calcium retention capacity
When the mitochondria are no longer able to retain calcium, calcium ion (Ca2+) exit the mitochondria through the permeability transition pore (PTP) opening of the mitochondria, we can measure the Ca2+ retention capacity by the susceptibility to mitochondrial PTP opening. After homogenizing the cerebellum tissue, we continuous measured response of calcium green 5-N fluorescence using a Spex Fluoromax 4 (HORIBA, Edsion, NJ, USA) at 37°C state four respiration (10-μg/mL oligomycin). After setting the background, and then Ca2+ (12.5 nM) was injected periodically at 506 nm (excitation wavelength) and 532 nm (emission wavelength) to measure and analyze the reaction. The maximum Ca2+ uptake capacity of the mitochondria before PTP opening (Ca2+ release) was represented as picomoles/sec/mg tissue weight.
Statistical analysis
We expressed data mean±standard error of the mean. IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. Statistical analysis was performed using one-way analysis of variance followed by Tukey post hoc test. Differences between groups were regarded as significant at P<0.05.
RESULTS
Protected cognitive performance
The radial arm maze test was used to confirm the establishment of VaD animal model and evaluate the effect of low-intensity exercise on the cognition dysfunction induced by CCH. We verified the well-established animal model through the results that the BCCAO group (3.5±0.4) was decreased correct performance compared to the sham group (6.4±0.5) (P<0.05) (Fig. 1A). BCCAO+ Ex group (5.3±0.6) showed significantly better performance as compared with the BCCAO group (P<0.05) (Fig. 1A). Error performance also showed the same result (Fig. 1B). We confirmed the improved cognitive performance by LITE.
Decreased loss of Purkinje cell
Calbindin D28k and parvalbumin are proteins that are participated in Ca2+ signaling, protects neuronal cells through reduced intracellular Ca2+ concentrations to maintain Ca2+ homeostasis, mainly locate in Purkinje cells and molecular layer interneurons in the cerebellum. The number of calbindin-positive Purkinje cells in the BCCAO+Ex group (41.50±2.66) was also significantly higher than the BCCAO group (31.00±1.67) (P<0.05) (Fig. 2A). The BCCAO+Ex group (29.83±1.01) showed significantly increased the number of parvalbumin-positive Purkinje cells compared with the BCCAO group (19.67±1.43) (P<0.05) (Fig. 2B). These results indicate that LITE protects the neuronal cell viability in the cerebellum.
Enhanced mitochondrial calcium retention capacity
One of major role of mitochondria is retention of calcium when they exceed the normal range of intracellular calcium level. In our study, the BCCAO+Ex group (751.28±52.97) showed significantly increased calcium retention capacity compared with the BCCAO group (424.19±28.46) (P<0.05) (Fig. 3). This result shows that low-intensity exercise has protective effect in the cerebellum by increasing calcium retention capacity.
DISCUSSION
The present study demonstrated that LITE protects the cognitive impairment induced by CCH through neuronal cell viability in the cerebellum and increasing the cerebellar mitochondrial calcium-binding capacity. The cerebellum has emerged a critical role in cognitive function as a connection between cerebrum and cerebellum, including cerebello-thalamo-cortical and cortico-ponto-cerebellar pathways (Palesi et al., 2017). It has been hypothesized that dysfunction of the cerebellum may play an important role in the pathology of VaD (Sui and Zhang, 2012). However, little research has been conducted in the cerebellum compared to the other brain regions to uncover the mechanism of CCH. The increase in studies of the cerebellum will be an opportunity for us to establish an essential basis for developing interventions that protect or restore cognitive health.
Most subjects who have suffered from CCH are old aged, so it is necessary to develop feasible and safe interventions for frail elderly (Buchner and Coleman, 1994). In this study, we applied the LITE to the CCH animal model. The low-intensity exercise showed a maximum oxygen uptake of 54% to 58% and better exercise adherence compared to the high-intensity exercise (Schefer and Talan, 1996). Moreover, the exercise reduced in injury risk and was generally more easily accessible to older adults (Marques-Aleixo et al., 2015). We observed the LITE prevented impaired cognitive performance induced by CCH. These results are consistent with previous studies showed that treadmill exercise ameliorated spatial memory performance using the 8-arm maze and Morris water maze test induced by CCH (Lee et al., 2017; Lee et al., 2018).
A previous study found that treadmill exercise in CCH-induced animal models has a preventive effect on the loss of Purkinje cells in the cerebellum via the suppression of glial cells and apoptosis (Lee et al., 2018). Our study enhanced the mitochondrial calcium retention capacity by LITE protected the loss of Purkinje cell in the cerebellum. Diminished Purkinje cells showed the spatial working memory deficit by acting minor or indirect role (Martin et al., 2004). Purkinje cells also express plenty of calcium-binding proteins, such as calbindin-D28k and parvalbumin. These proteins have engaged the regulation of cell cycle and intracellular calcium concentration related to apoptosis (Zhao et al., 2008). One of the main causes of loss of Purkinje cell has been known the mitochondrial dysfunction.
Mitochondrial dysfunction is regarded as one of the major causes of neuronal injury in VaD induced by CCH (Venkat et al., 2015). Dysfunction of mitochondria represents diminished production of adenosine triphosphate, impaired calcium buffering capacity, and increased reactive oxygen species (Zorov et al., 2014). The mitochondrial PTP and forms a transmembrane pore that is large enough to allow release of cytochrome c. Mitochondrial calcium retention capacity was checked by measuring the PTP opening of mitochondria. Notably, the decline of calcium retention capacity affects the mitochondrial calcium homeostasis, which results in apoptosis through the caspase signaling cascade (Hajnóczky et al., 2006). Intracellular calcium overload activates the caspase that the proteolytic activity of caspases has the biochemically vital role of apoptosis (Zhivotovsky and Orrenius, 2011). In this study, we observed the mitochondrial calcium retention capacity in VaD group was decreased compared to sham group. Exercise improved the mitochondrial calcium retention capacity in the brain (Seo et al., 2019). Especially, low-intensity exercise improved physical and cognitive health (Tse et al., 2015). In accord with our result, the previous research showed that physical exercise increased manganese-dependent superoxide dismutase activity and calcium retention capacity in the cerebellum (Marques-Aleixo et al., 2015).
In conclusion, we showed that LITE might exert a neuroprotective effect against the loss of Purkinje cells via increasing the mitochondrial calcium retention capacity in the cerebellum. Therefore, LITE may have potential as a therapeutic intervention strategy for the attenuation of cognitive impairment in patients with CCH.