INTRODUCTION
Pelvic floor muscle exercise (PFME), also known as pelvic floor muscle training, pelvic floor physiotherapy or pelvic floor rehabilitation, is a mainstay of behavioral treatment for urinary incontinence and overactive bladder symptoms, such as urgency (Lee et al., 2017). PFME is defined as exercise to improve pelvic floor muscle strength, power, endurance, relaxation, or a combination of these parameters (Ahlund et al., 2013). PFME is effective in all type of urinary incontinence. In stress urinary incontinence, the mechanism is characterized by use a conscious contraction before or during increases in intra-abdominal pressure, and a building up a structural support (Di Benedetto et al., 2008). In urgency urinary incontinence, the biological rationale is that an involuntary detrusor contraction can be reflexively or voluntarily inhibited by pelvic floor muscle activation, therefore, voluntary pelvic floor muscle contraction may be used to control urgency (Bientinesi et al., 2020; Di Benedetto et al., 2008).
PFME was first introduced by Margaret Morris in 1936, to instruct women how to exercise vaginal muscles with the aim of strengthening the muscles and preventing stress urinary incontinence. However, PFME was first popularized by Arnold Kegel, a gynecologist who suggested that stress urinary incontinence was due to a lack of awareness of function and coordination of pelvic floor muscles (Kegel, 1948). Dr. Kegel also proposed that patients could reduce their stress urinary incontinence through consecutive pelvic muscle contractions to increase strength (Kegel, 1948; Kegel, 1956). Over time, this exercise has evolved as a behavioral and physical therapy, into a conservative treatment for urinary incontinence.
PFME is an exercise that involves the understanding of pelvic floor muscle activation and the pursuit of a repeated PFME program over time. Because effectiveness depends on the patients’ adherence during the intervention and afterwards in the maintenance phase, a better understanding of adherence mechanisms and how they can be promoted is of major importance. This present review paper provides an overview of clinical application of PFME as a behavioral therapy for urinary incontinence. In addition, this article presents recent evidence on PFME in the clinical and research settings.
ANATOMY OF PELVIC FLOOR MUSCLES
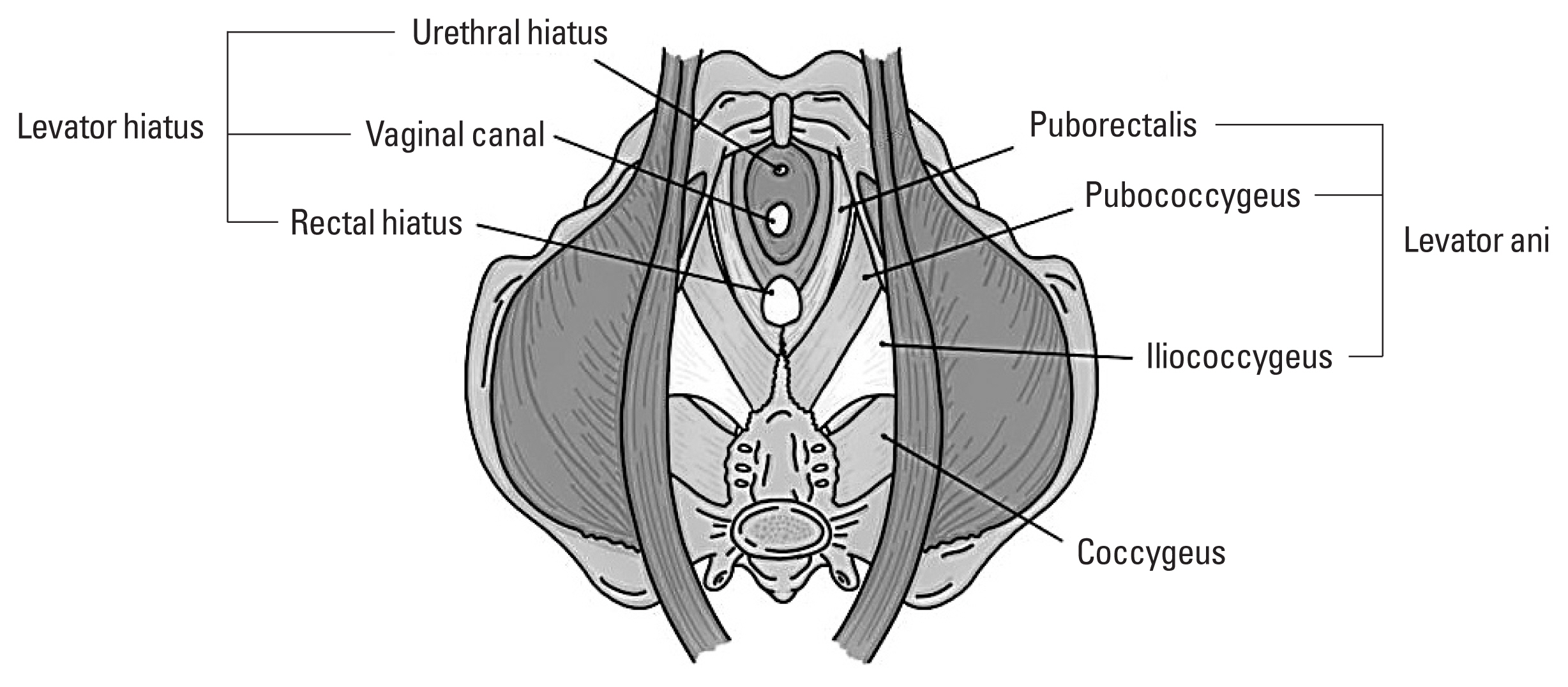
The pelvic floor refers to a group of muscles that support the organs in the pelvis. This is composed of the pelvic diaphragm, which extends from the symphysis pubis anteriorly to the coccyx posteriorly, forming a hammock-like structure which supports pelvic organs. The pelvic floor muscles are composed of levator ani muscles including puborectalis, pubococcygeus and iliococcygeus muscles, and coccygeus muscles. The levator ani muscles play an essential role in support of the pelvic organs and are innervated by the fourth sacral nerve.
The puborectalis muscle arises from the back of the pubis and form a U-shaped loop behind the rectum that slings the anorectal junction to the symphysis pubis. The pubococcygeus muscle arises from the posterior portion of the superior pubic ramus and attaches to the anococcygeal and the superior surface of the coccyx. The iliococcygeus muscle arises from the ischial spine and inserts on the anococcygeal raphe and the coccyx. The name of each muscle of its components is derived from its attachments (Newman, 2014) (Fig. 1). The coccygeus and iliococcygeus were previously thought to be innervated by divisions of the pudendal nerve, inferior rectal nerve, and perineal nerve (Kim et al., 2020b). However, recent studies show the innervation solely from the levator ani nerve originating from S3,4,5 and traveling medial to the ischial spine and the arcus tendineus levator ani.
The levator plate is made by the fusion of the levator ani muscles and the sustained resting tone of the pelvic floor muscles supports pelvic organ, resists increase in abdominal pressure, and is essential in control of urinary and fecal continence (Kim et al., 2021). With loss of tone from pelvic floor muscles or nerve injury, there is relaxation in the urogenital hiatus, which diminishes the horizontal orientation of the levator plate.
EVALUATIONS OF PELVIC FLOOR MUSCLES
Adequacy of pelvic floor muscle contraction can be assessed during the pelvic examination. Pelvic floor muscle function is assessed by several methods, including visual observation, digital palpation, electromyography (EMG), manometry, or ultrasound. Prior to PFME, an evaluation of the pelvic floor muscle can provide valuable baseline standard about strength, coordination, and control. Visual observation can be used to assess a correct pelvic floor muscle contraction, which is seen by perineal elevation, or an incorrect contraction observed by perineal descent in women (Bo et al., 2017). Digital palpation, such as the Brink score and the Laycock power, endurance, repetitions, fast, every, contraction, timed (PERFECT) assessment scheme is the most used method in clinical practice (Laycock and Jerwood, 2001; Newman and Wein, 2013; Newman et al., 2018). The Brink score employs a 4-point scale to assess the contraction pressure, vertical displacement, and endurance of squeeze (Brink et al., 1989). The Laycock PERFECT assessment scheme uses a 6-point scale to score strength and endurance and the number of repetitions and fast contractions (Laycock and Jerwood, 2001). It is important to know that women may use the wrong muscles, straining down or performing a Valsalva maneuver, when asked to contract the pelvic floor muscle. The examiner should check whether gluteal or abdominal muscles contract.
When assessing pelvic floor muscle strength, clinicians should use three criteria (Brink et al., 1989). Pressure, duration, and alteration in position. Note the results of the amount of pressure or strength of the muscle contraction and the number of seconds that the examiner feels the muscle contraction. If the patient has an overactive pelvic floor muscle, the degree should be noted. Overactive pelvic floor muscle is defined as no relaxing or contracting when relaxation is needed, such as micturition (Messelink et al., 2005). Evaluation of the pelvic floor muscle in men and women can also be performed by assessing anal sphincter contraction and tone. To assess the strength of the sphincter muscle, the patient is asked to tighten the rectum. The examiner should feel a griping or pulling in around the finger circumference. Resting sphincter tone can be noted as weak, moderate, or strong.
INSTRUCTIONS OF PELVIC FLOOR MUSCLES EXERCISE
Patients have better outcomes with regular PFME and proper technique. The first step in PFME is to instruct the patient how to identify the pelvic floor muscles and to contract and relax them. This can be done using verbal feedback based on digital assessment and visual or auditory biofeedback or electrical stimulation (Cho and Kim, 2020; Newman, 2014; Newman and Wein, 2013). There are four main instructions for patients. First, contract muscles around examiner’s finger and try to pull up and in. Second, imagine trying to prevent the passing of bowel gas by tightening the ring of muscles around the anus without tensing the muscles of legs or buttocks. A closing and lifting sensation should be felt. Third, imagine moving the penis up and down without moving any other part of the body for men. Last, should feel vagina and rectum pull up and in for women.
One problem commonly encountered in teaching PFME is muscle isolation. More than one-third of women report not feeling confident about their ability to do a pelvic floor muscle contraction correctly (Chiarelli et al., 2003). Patients tend to recruit other muscles, such as the rectus abdominis muscles or gluteal muscles (Newman et al., 2018). In addition, they substitute a straining-down Valsalva maneuver, which threatens to worsen the conditions (Sampselle and DeLancey, 1992). Therefore, it is important to observe for the use of other muscles and to help patients to contract pelvic floor muscles selectively while relaxing ancillary muscles. It is also important to verify that patients have identified and can contract the pelvic floor muscles properly before initiating a PFME regimen. Inability to identify pelvic floor muscles or not exercising correctly may be the most common reason for poor outcomes with PFME. For patients with difficulty identifying the proper muscles, supplemental therapies can help those to perform PFME properly.
SUPPLEMENTAL THERAPIES FOR PELVIC FLOOR MUSCLES EXERCISE
Supervised PFME
Patients who have difficulty performing PFME or have no improvement with unsupervised PFME may benefit from referral to a continence nurse practitioner or pelvic floor physical therapist (Fitz et al., 2020). PFMEs are most effective with specific instruction by health professionals and regular performance by motivated patients. It is not clear how much health care supervision is optimal (Dumoulin and Hay-Smith, 2010; Hay-Smith et al., 2011). Both group and individual sessions appear to be helpful (Dumoulin et al., 2020).
Vaginal cones
Weighted vaginal cones were developed as a method for helping patients identify and control their pelvic floor muscles and to support adherence to PFME. These may be preferable for women who have insufficient time or resources to dedicate to supervised physical therapy or biofeedback. The woman inserts the cone in her vagina and uses pelvic muscle contractions to hold it in place during activity. When a weighted cone is placed in the vagina while standing, the sensation of “losing the cone” provides strong sensory feedback that prompts the pelvic floor muscle to contract to prevent it from slipping out. Vaginal cones have increased efficacy over no active treatment but inconclusive evidence that they provide increased efficacy over standard PFME (Herbison and Dean, 2013).
Biofeedback
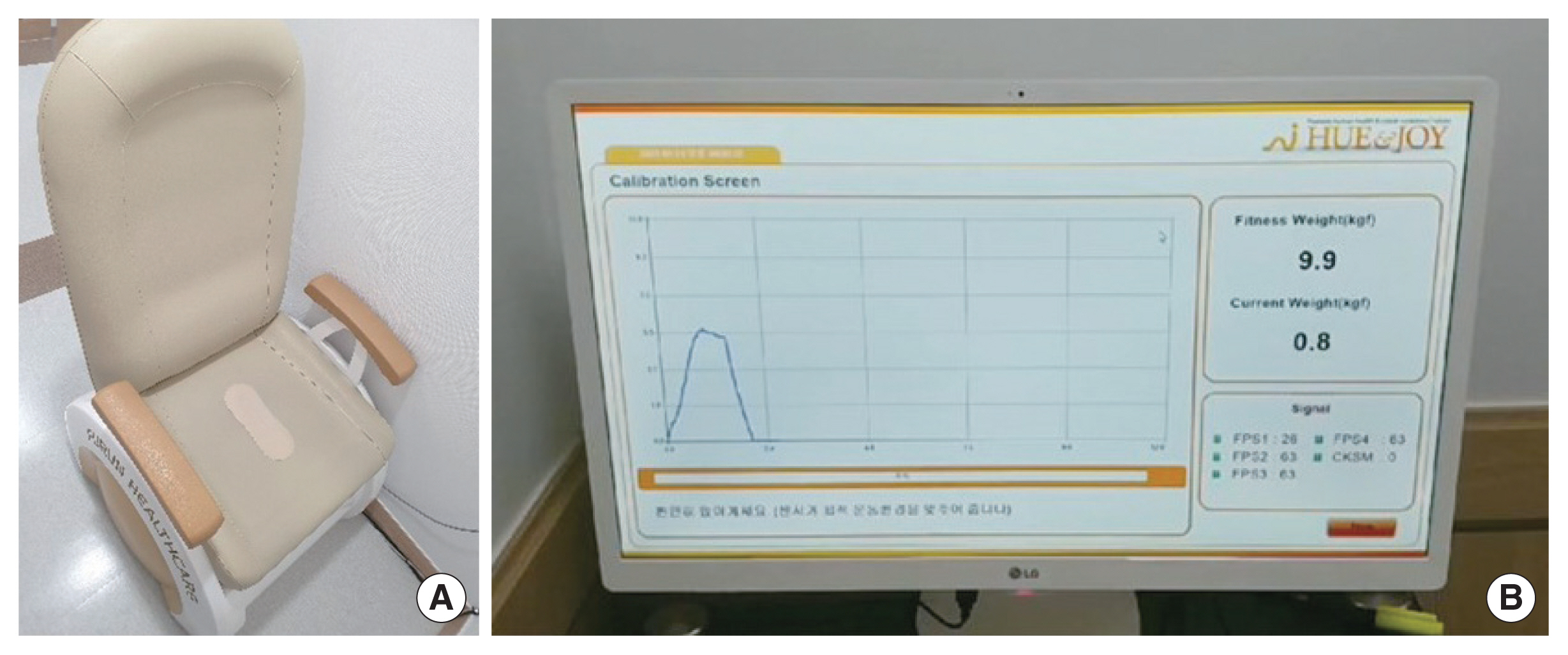
Biofeedback therapy is a technique in which neuromuscular and autonomic activity are monitored, amplified, and conveyed to the patient as visual or acoustic signals (Newman, 2014). Biofeedback as a supplement to PFME is particularly useful in patients who are unable to properly isolate the pelvic floor or use accessory muscles during pelvic floor contractions. This modality involves placement of a vaginal pressure sensor within the vagina that measures pressure and provides an audible or visual feedback of strength of pelvic floor contraction. Augmented versions also use abdominal and perineal EMG recordings to demonstrate improper contraction of abdominal and gluteal muscles.
Biofeedback computer equipment records pelvic floor muscle activity, the muscle contraction, relaxation, and strength. It produces computer-generated graphs that are displayed on a monitor (Fig. 2). Biofeedback helps patients find their pelvic muscles by showing changes when they squeeze or tighten the right muscle. Patients can use biofeedback equipment to reach a new level of strength in pelvic floor muscles (Newman, 2014). Biofeedback is often used in conjunction with supervised PFME. Women who received PFME with biofeedback, were more likely to have improvement of urinary incontinence (relative risk, 0.75; 95% confidence interval, 0.66–0.86), compared with women who received PFME alone (Herderschee et al., 2011).
Biofeedback is also used in patients with bowel dysfunction and fecal incontinence. The goal for these patients is to strengthen the anal sphincter to restore a normal pattern of defecation. For dyssynergic defecation and loss of stool, the goal of neuromuscular training is two-fold to correct the dyssynergia or incoordination of the abdominal, rectal, puborectalis, and anal sphincter muscles to achieve a normal and complete evacuation, and to enhance rectal sensory perception in patients with impaired rectal sensation (Markland et al., 2008).
Biofeedback parameters include measurement of resting tone, strength, power, contractility, and endurance (Table 1) (Newman, 2014). At least a 5-sec muscle relaxation period should be used between each contraction because easily fatigable muscles need a chance to recover, without permitting excessive rest periods for strong muscles. EMG provides electrical activity information on the ability of the pelvic floor muscle to contract, and manometry quantifies pelvic floor muscle strength (Newman, 2014).
Electrical stimulation
Pelvic floor muscle electrical stimulation with a nonimplanted device involves the application of a low grade of electrical stimulation to the pelvic floor muscles to stimulate a contraction in patients with different types of urinary incontinence or symptoms of urgency, frequency, and nocturia. Electrical Stimulation has a contraction of pelvic floor muscles and inhibition of unwanted detrusor contractions (Schreiner et al., 2013). Long-term or chronic electrical stimulation delivered below the sensory threshold aiming at detrusor inhibition by afferent pudendal nerve stimulation. On the other hand, maximal electrical stimulation, using a high-intensity stimulus (just below the pain threshold), aims to improve urethral closure by direct and reflexogenic contraction of striated peri-urethral musculature. The device is placed in the vagina or anus and provides a small electrical current that stimulates the pelvic floor muscles to contract, aiding the patient in identification and isolation of the proper muscles. Intravaginal electrical stimulation improved continence rates for women with stress, urgency, or mixed urinary incontinence compared with sham stimulation. However, it does not appear to enhance outcomes over those obtained through PFME alone or PFME with biofeedback.
Mobile applications
While both free and paid applications exist to aid PFME and address urinary incontinence in females, their efficacy has not been well studied. There are 139 mobile applications targeting female stress urinary incontinence and 20 applications met criteria for review (Ho et al., 2021). Of the 20 applications, only one had been trialed and verified in the literature with reported efficacy (Asklund et al., 2017). Based on the Mobile App Rating Scale (MARS) tool (0 to 5 rating scale), the median MARS for the 20 applications was 3.6, with a range of 2.7 to 4.1 (Ho et al., 2021).
REGIMENS OF PELVIC FLOOR MUSCLES EXERCISE
Once patients demonstrate the ability to properly contract and relax the pelvic floor muscles, they are given instructions for daily practice and exercise. The purpose of daily exercise is to increase muscle strength and to enhance motor skills through practice. The basic regimen consists of 3 sets of 8 to 12 contractions sustained for 8 to 10 sec each, performed 3 times a day. Patients should try to do this every day and continue for at least 15 to 20 weeks. Specific exercise regimens vary considerably in frequency and intensity, and the ideal exercise regimen has not yet been determined (Newman et al., 2018). However, good results have been achieved in several trials using 45 to 60 paired contractions and relaxations per day. Some clinicians use an exercise prescription to prescribe the daily exercise program (Newman, 2014; Newman and Wein, 2013).
One approach is to recommend a short, quick exercise of 1- to 2-sec contractions, followed by long, sustained exercise of 5–10 sec. The patient is encouraged to aim for a high level of concentrated effort with each pelvic floor muscle contraction because greater contraction intensity is associated with improvement in pelvic floor muscle strength. It is equally important to relax the pelvic floor muscle completely between each contraction. Each exercise consists of a muscle contraction followed by a period of relaxation using a 1:1 or 1:2 ratios. This allows the muscles to recover between contractions and facilitates optimal strength building.
It is usually recommended that patients space the exercises throughout the day, typically in 2 to 5 sessions per day to avoid muscle fatigue. Exercising while in the prone position is often recommended at first, because it is the least challenging. However, it is important for patients to progress sitting or standing positions with time so that they become comfortable and skilled using their muscles to avoid urinary incontinence in any position (Newman et al., 2018) (Table 2).
PREVENTING STRESS URINARY INCONTINENCE
The goal of behavioral treatment for stress urinary incontinence is to teach patients how to prevent urine loss in daily life by occluding the urethra using active contraction of pelvic floor muscles (Miller et al., 1998). A careful medical history or examination of frequency volume chart can alert the provider and patient of the circumstances during which each individual patient commonly experiences urine loss. Patients then learn to anticipate these activities and prevent leakage by a volitional contraction of the pelvic floor muscle to occlude the urethra before and during coughing, lifting, or any other physical activities that has precipitated urine leakage. When an anticipatory pelvic floor muscle contraction technique was used, immediate reduction in the volume of urine leakage with a cough was demonstrated (Miller et al., 1998; Miller et al., 2008). If initial PFME treatments are not sufficient for patients with stress urinary incontinence, patients should be referred for additional treatment options including surgery. For women who desire more rapid and definitive treatment who are willing to accept risks of surgery, the mid-urethral sling offers higher success rates than conservative therapy (Kim et al., 2020a).
PELVIC FLOOR MUSCLES EXERCISE FOR MEN
Male stress urinary incontinence is reported to occur in 1%–60% of patients following radical prostatectomy. To date, artificial urinary sphincter implantation has been the standard treatment (Ko et al., 2019). However, the PFME is the most recommended initial conservative treatment for urinary incontinence after radical prostatectomy (Ha and Yoo, 2019). Trials of PFME for post-prostatectomy urinary incontinence show that PFME reduces urinary incontinence in the first 3 months after surgery (Campbell et al., 2012). Some reviews demonstrated that PFME is better than no treatment, because it can reduce urinary incontinence episodes. Another study showed that an early PFME after radical prostatectomy significantly reduces continence recovery time (Filocamo et al., 2005). The rationale of PFME is that pelvic floor muscle contraction may improve the strength of the external urethral sphincter during the raises of intra-abdominal pressure. In pelvic floor muscle electrical stimulation therapy, less work has been done examining electrical stimulation in men, and there is little evidence to suggest that electrical stimulation improves outcomes of PFME, with or without biofeedback, but it may promote the improvement of urinary incontinence after prostatectomy (Berghmans et al., 2013).
PELVIC FLOOR MUSCLES EXERCISE IN FRAIL OR COGNITIVE IMPAIRMENT OLD PEOPLE
Older people may be considered wealthy or frail. In contrast to wealthy, the term frail older persons define the people over the age of 65 years with a clinical presentation of impaired physical activity, mobility, balance, muscle strength, motor processing, cognition, and feelings of fatigue. However, frailty is not synonymous with disability and comorbidity, even if frail people usually have multiple chronic medical conditions, take multiple drugs, and have difficulties in the personal activities of daily life and a high risk of intercurrent diseases, hospitalization, and death. This is very important to make therapeutic plans for older people. No differences exist among the PFME for adult and wealthy older people. However, initial management should be individualized and influenced by treatment goals, preferences, and estimated remaining life expectancy, as the most likely clinical diagnosis. In treating urinary incontinence of the frail elderly, PFME is considered as fist-line therapy (Na and Cho, 2020). In association with other conservative measures, it has a relevant role in the improvement of quality of life. Age is no barrier to the benefits of PFME (Lee et al., 2017; Na and Cho, 2020).
Management of urinary incontinence in older patients with cognitive impairment is based on multiple factors, including cognitive state, functional impairment, concurrent comorbidities, and polypharmacy. Behavioral therapy under caregiver support represents appropriate treatment strategy for urinary incontinence in these patients (Na and Cho, 2020). Lee et al. reported that the effects of PFME in elderly women with mild cognitive impairment or Alzheimer disease were like those observed in elderly women with normal cognitive function in a previous report (Lee et al., 2017; Sherburn et al., 2011). Regular supervision by an expert is important for improvements in urinary incontinence and the patient’s satisfaction. Moreover, regular evaluation and feedback by the physiotherapist helps to build a patient’s sense of efficacy regarding PFME and to maintain adherence to the treatment (Alewijnse et al., 2001). Therefore, regular check-ups and assurances by the expert therapist in their study contributed to the continuation of PFME and significant improvements in urinary incontinence in elderly women with cognitive impairment (Lee et al., 2017).
CLINICAL TRIALS OF PFME
Most samples were drawn from community-dwelling populations. Most trials involved 12 weeks of intervention (Pereira et al., 2011), but some were of 6 weeks or 8 weeks duration (Kargar Jahromi et al., 2015). Most involved supervised PFME accompanied by a regimen of home-based exercises by a physiotherapist or nurse. In several trials, PFME was delivered on an individual basis (Celiker Tosun et al., 2015; McLean et al., 2013; Pereira et al., 2011), while others were conducted in a group class (Kargar Jahromi et al., 2015; Kim et al., 2011). Some trials were restricted to PFME with or without functional pelvic floor muscle contraction to prevent stress urinary incontinence episodes (McLean et al., 2013; Pereira et al., 2011), while in others, PFME was embedded in a multicomponent program with other behavioral or exercise components. One trial included PFME in a broader program of general physical exercise (Kim et al., 2011).
PFME is effective as a stand-alone therapy, as part of multicomponent therapies embedding PFME with concomitant behavioral strategies, lifestyle changes, and as part of more general physical exercise programs to improve physical function in older women. Results expand the evidence base to include PFME implemented by mobile technology, with a potentially broader reach, cost savings, and impact on rural health. Benefits are shown across age cohorts and urinary incontinence type, in various cultural contexts, using several different training regimens, and assessed by multiple outcome measures.
EVIDENCE FOR PFME
The PFME is an established treatment supported by a very large body of evidence (Dumoulin et al., 2018). A Cochrane review concluded that PFMEs were effective for stress and mixed urinary incontinence (Dumoulin et al., 2014; Dumoulin et al., 2018) and can reduce urgency, but women with pure stress urinary incontinence may have better outcomes (Bø and Herbert, 2013). PFME is better than no treatment, placebo, or an inactive control treatment for women with stress, urgency, and mixed urinary incontinence (Ayeleke et al., 2013; Dumoulin et al., 2018). Cure rates for PFME range from 16% to 27% and improvement rates from 48% to 80.7%. Evidence is lacking regarding the best approach to PFME, but there is consensus that supervised PFME is more effective than unsupervised programs (Hay-Smith et al., 2011; Hay-Smith et al., 2012; Lee et al., 2017), although the degree and type of supervision needed are uncertain. The effect of PFME in women with stress urinary incontinence does not seem to decrease with age. In trials of older women, primary and secondary outcome measures were comparable with those in trials focused on younger women. Thus, age should not be a deterrent to PFME (Betschart et al., 2013). Based on this evidence, supervised PFME should be offered as a first-line conservative therapy for women of all ages with stress, urgency, or mixed urinary incontinence (Lee et al., 2017).
CONCLUSIONS
This review article provides clinical application of PFME as a behavioral treatment for urinary incontinence. PFME strengthen the pelvic floor muscle to provide a backboard for the urethra to compress on. Clinicians should understand pelvic floor muscle anatomy, evaluation, and regimen. In addition, it is necessary for clinician to teach patients how to train the pelvic floor muscles to relieve the symptoms. PFME is a unique exercise that take time to master, but with repeated training, most patients are successful.