AbstractBlood flow restriction (BFR) resistance exercise has been advocated as an alternative approach for improving muscle strength in patients undergoing musculoskeletal rehabilitation. The present study aimed to evaluate the effectiveness of a 4-week supervised rehabilitation (R) with and without BFR on muscle strength, cross-sectional area (CSA), dynamic balance, and functional performance in athletes with chronic ankle instability (CAI). A total of 16 collegiate athletes with CAI participated in this study. They were randomly assigned to the BFR+R group (n=8) or the R group (n=8). Both groups underwent supervised rehabilitation 3 times weekly for 4 consecutive weeks. Additionally, the BFR+R group was applied with a cuff around the proximal thigh at 80% arterial occlusion pressure in addition to the traditional rehabilitation program, whereas the R group received the sham BFR only. Before and after 4 weeks of intervention, isokinetic muscle strength, CSA, Y-balance test, and side hop test (SHT) were measured. Following a 4-week intervention, the BFR+R group exhibited significant improvements in muscle strength of ankle plantarflexor and evertor, CSA of fibularis longus, and SHT timed performance compared with prior training and the R group (all, P<0.05). However, no significant difference was observed on dynamic balance among the groups. The present finding indicated that a 4-week supervised rehabilitation combined with BFR is more effective in improving muscle strength and size and functional performance compared with the traditional rehabilitation alone. This information could have implications for physical therapists and clinician in developing and designing a rehabilitation program for athletes with CAI.
INTRODUCTIONLateral ankle sprain (LAS) is a common sports injury experienced by many athletes, including rugby, football, volleyball, handball, and basketball players (Valderrabano et al., 2006). In addition, approximately 40% of individuals encounter an initial ankle sprain may progress to chronic ankle instability (CAI) (Gerber et al., 1998). CAI is well documented to have a detrimental effect on sport performance. It causes athletes to be away from training camps and prevents them from participating in physical activities (Hubbard-Turner and Turner, 2015). In general, CAI is characterized by repetitive episodes or perceptions of the ankle giving way, which is accompanied with persistent symptoms such as pain, muscle weakness, decreased ankle range of motion (ROM), diminished self-reported function, and recurrent ankle sprains (Hertel and Corbett, 2019). At present, the mechanisms underlying CAI are not fully understood (Hertel, 2002; Hertel and Corbett, 2019). Mechanical insufficiencies induced by ligamentous laxity can be one factor. Another mechanism involves functional insufficiencies, including impairments in proprioception, neuromuscular control, strength, and postural control (Delahunt, 2007). Thus, a traditional rehabilitation program is primarily focused on strength, neuromuscular training, and proprioceptive training (Hall et al., 2018). Several ankle rehabilitation programs have been developed for treating and managing CAI (Hale et al., 2007; Hall et al., 2018; McKeon and Wikstrom, 2018). However, most rehabilitation programs have been limited by which a heavy load or resistance could not be applied; therefore, they could not fully activate muscle contraction and metabolic stress, which provide an important signal for muscle strength and hypertrophy (Boudreau et al., 2007).
Blood flow restriction (BFR) resistance exercise has emerged as a new rehabilitation for patients with postoperative surgery, such as knee arthroscopy, anterior cruciate ligament reconstruction, and Achilles’ tendon rupture, including patients with nonoperative musculoskeletal deficits, for example, knee osteoarthritis, patellofemoral pain syndrome, anterior knee pain, or CAI (Burkhardt et al., 2021; Fujita et al., 2007; Killinger et al., 2020). This novel rehabilitation strategy involves the application of an inflatable cuff or tourniquet around the limb, which maintains arterial inflow while occluding venous return during exercise, resulting in local hypoxia (Loenneke et al., 2012). In addition, BFR has consistently shown to enhance training adaptation in muscle strength and hypertrophy when combined with low-load resistance exercise at 20%–30% of one repetition maximum for 2 weeks and functional exercises, such as walking for 3 weeks (Abe et al., 2006; Scott et al., 2016). BFR training can increase muscle strength and hypertrophy through inducing local hypoxia, metabolic stress, and the recruitment of high-threshold motor units or type 2 muscle fiber (Lauver et al., 2017; Suga et al., 2012; Yanagisawa and Sanomura, 2017). Moreover, BFR training can improve the strength of remote muscles through increasing the amplitude of motor-evoked potential in corticospinal excitability, which alters motor output to distal BFR area (Patterson and Ferguson, 2011). Most recent studies on individuals with CAI demonstrated great fibularis longus and tibialis anterior muscle activations during submaximal isometric resistance exercise with BFR compared with the control (Killinger et al., 2020). Furthermore, Burkhardt et al. (2021) have recently reported large-to-small increases in muscle activation of the vastus lateralis and soleus muscles and increases in postural instability and exertion during dynamic balance exercise combined with BFR in individuals with CAI.
Previous studies have focused primarily on the use of BFR during a specific exercise such as isometric exercise and dynamic balance exercise; however, this approach appears to be less clinically relevant because of the multifaceted nature of CAI. Furthermore, evidence shows that performing multiple rehabilitation exercises by combining balance training, strengthening, and proprioceptive training may be more effective in improving postural control and preventing recurrent injury (Emery and Meeuwisse, 2010; Hale et al., 2007; Jaber et al., 2018). To the best of our knowledge, no study has examined the efficacy of a traditional rehabilitation combined with BFR on clinical outcome measures such as muscle strength, dynamic balance, or functional performance in athletes with CAI. Therefore, this study aimed to evaluate the effectiveness of the BFR+R versus the R program on muscle strength, cross-sectional area (CSA), dynamic balance, and functional performance in athletes with CAI. This study hypothesized that the BFR+R program would further improve muscle strength, CSA, dynamic balance, and functional performance in CAI athletes compared with the R program alone.
MATERIALS AND METHODSParticipantsA total of 16 athletes with CAI (mean age, 22±1.03 years; weight, 62.69±8.68 kg; height, 171.81±7.85 cm; and body mass index, 21.11±1.19 kg/m2) participated in this study. They were matched by age, sex, and Cumberland Ankle Instability Tool (CAIT) scores and were randomly allocated to either the BFR+R group (n=8) or the R group (n=8) (Fig. 1). All participants were varsity athletes (rugby, football, volleyball, handball, and basketball), who were members of the Burapha University athletic team, Thailand. Prior to the start of the study, participants were asked to complete the questionnaires, attend all assessment sessions, and be diagnosed by a registered physical therapist. Participants were included in the study if they reported a history of unilateral LAS, which occurred at least 12 months prior to study enrolment, a history self-reported giving way and/or feelings of ankle instability of the involved ankle during activities of daily living and/or sporting activities for at least 6 months, and a score ≤24/30 on the CAIT. The exclusion criteria consisted of a history of bilateral ankle instability; pathological joint laxity (a positive result on the talar tilt test or anterior drawer test); a history of ankle fracture; surgery of the hip, knee, and ankle; and a history of the musculoskeletal disorders. All participants were informed of the procedures, benefits, and risks of the study, and they obtained informed consent before enrolling in the study. This study was approved by the Research Ethics Review Committee for Research Involving Human Research Participants, Chulalongkorn University, Thailand (COA NO. 017/2021) and was in accordance with the standards set by the Declaration of Helsinki.
Study designThis study was a single-blinded randomized parallel controlled design. The study involved two testing sessions before (pretest) and after (posttest) a 4-week intervention. The data were collected from March to June 2021. Before the study, participants were familiar with all the testing apparatus and exercise protocol. On the testing day, participants reported to the laboratory in the morning (9.00–12.00 a.m.). Upon arrival, participants rested for 10 min, followed by the isokinetic strength test (Gribble and Robinson, 2009), muscle CSA measurement (Lobo et al., 2016), dynamic balance (Gribble and Hertel, 2003), and side hop test (SHT) (Linens et al., 2014; Madsen et al., 2018). All testing sessions were performed at the same time of day with similar environmental conditions (temperature ~24°C–25°C, relative humidity ~40%–44%). Participants were instructed to avoid any kind of strenuous activities 24 hr before the measurement. They were also asked to abstain from caffeine intake and consume a light meal 2–3 hr before the measurement.
Sample size calculationThis study was part of a larger investigation of the efficacy of BFR training combined with a rehabilitation program on neuromuscular function and balance in athletes with CAI. A priori sample size was calculated based on our pilot data for the average relative peak torque (concentric/concentric) of ankle plantar flexors with an estimated effect size of 1.61, an alpha level of 0.05, and desired power of 80% using G*Power v 3.1.9.2. Based on the sample size estimation, a minimum of 16 participants (n=8/group) were required.
BFR trainingA pneumatic occlusion cuff of 10-cm width and 75-cm length was used for BFR training. The participants were asked to lie in the supine position and rest for 5 min. Then, a cuff of appropriate size was wrapped around the proximal thigh of participant’s CAI limb, and a handheld portable doppler probe (SD3 Vascular, Edan Instrument, Inc., Shenzhen, China) was used to measure participant’s posterior tibial artery at the involved side by inflating the cuff to 80% of each participant’s personalized arterial occlusion pressure (Lixandrao et al., 2015). For the R group, participants wore a BFR cuff similar to that of the experimental group, but the BFR cuff had no inflation.
Rehabilitation interventionThe program began with a 5-min warm-up, consisting of dynamic stretching of lower limb muscles, followed by a 30-min supervised rehabilitation program (Table 1). Exercise progression included a single-leg heel raised with weight, single-leg squats, single-limb stance on Bosu ball, and double-limb stance with throwing, catching on Bosu, and Y-balance test (YBT) functional reaching, which have been previously described (Jaber et al., 2018). The BFR cuff of the participants in the BFR+R group remained inflated throughout the duration of a rehabilitation program and deflated during rests between trials. Participants completed a supervised rehabilitation program 3 times weekly for 4 consecutive weeks. All training sessions were conducted in a laboratory under a supervision of the same registered physical therapist. Participants did not participate in any other exercise except for the exercise programs provided in this study.
Outcome measurementsAn isokinetic dynamometer (CON-TREX MJ, TP module, Physiomed Elektromedizin AG, Schnaittach, Germany) was used to determine the average peak torque to body weight ratio (concentric/concentric) at the angular velocity of 60°/sec for hip extensor, abductor, ankle dorsiflexor/plantarflexor, and ankle evertor/invertor muscles as previously described (Gribble and Robinson, 2009). The dynamometer was calibrated before testing each participant. Three practice repetitions of submaximal and three practice trials of maximal contractions were performed to allow familiarization with the testing protocol. After a 2-min rest, three continuous repetitions throughout the active ROM of maximal concentric contraction were performed by the reciprocal muscles of a joint in a given movement direction. A 5-min rest interval was considered between each test condition to allow adequate recovery among contractions. Each participant received positive verbal encouragement during testing to ensure that a maximal effort was attained. For each testing, three maximal contractions for the involved side of the hip extensor, abductor, ankle dorsiflexor/plantarflexor, and ankle evertor/invertor were recorded using the Physiomed Software Package. The highest peak torque values were recorded for each of the three repetitions, and the average was considered as average peak torque to body weight ratio (Negahban et al., 2013). In the present study, the intraclass correlation coefficient (ICC) for the hip extensor, abductor, ankle dorsiflexor, ankle plantarflexor, ankle evertor, and ankle invertor was 0.96, 0.92, 0.98, 0.87, 0.97, and 0.96, respectively.
The CSA of the fibularis longus muscle was measured using a B-mode diagnostic ultrasound system (M5 series, Shenzhen Mindray Bio-Medical Electronics Co., Shenzhen, China) with a 3.5- to 13-MHz linear transducer, linear array probe with 38-mm probe surface length, and frequency of 10 MHz (7L4s, Shenzhen Mindray Bio-Medical Electronics Co.) (Lobo et al., 2016). Briefly, participants were in the supine position with approximately 20°–45° knee flexion of the injured side. The probe location was marked at the midpoint between the upper part of the fibular head and the inferior border of the fibular malleolus of the injured side. A probe with ample gel was placed perpendicular to the drawn line, and a clear image of the fibularis longus muscle was scanned, which was inside the connective tissue of the injured side. The image was captured, and the average CSA of three repetitions were recorded for analysis. Image processing and analysis were performed by tracing the muscle border, using image J software version 1.51 (Wayne Rasband, NIH, Bethesda, MD, USA). In the present study, the results indicated good reliability, with an ICC of 0.98.
The YBT was used for assessing a dynamic balance. The participants were standing on a single leg with the involved limb, with hands on hips, and placed at the center of the grid with three lines in the specific anterior, posteromedial, and posterolateral directions. All participants were remained with the hallux at the point of intersection in the anterior direction. Then, the participants were instructed to place their heel at the intersection of the three strips of tape in the posteromedial and posterolateral directions (Plisky et al., 2009). The participants were instructed to reach out as far as possible with his or her leg in the target direction without touching the tape or losing balance. Thereafter, participants return to the starting position and then repeat the motion for continued practice. A 30-sec rest between tests was allowed for each side. The starting position was random to avoid a learning effect. The lower limb length (LL) was measured from the anterior superior iliac spine to distal end of the medial malleolus in supine lying position. The YBT composite score was calculated by dividing the sum of the three reach distances in the anterior, posteromedial, and posterolateral directions by 3 times the LL of the individual and then multiplied by 100: [(anterior+posteromedial+posterolateral)/(LL×3)]×100. The results were expressed in percentage (Gribble and Hertel, 2003). Reach distances were divided by leg length and multiplied by 100 to calculate a dependent variable, which represented the reach distance as a percentage of leg length.
The SHT was used as a tool for assessing the functional limitation of the involved limb. Participants stood on the test leg and then jumped from side to side as fast as possible between two parallel lines placed 30 cm apart for a total of 10 times. The total time taken to complete 10 repetitions was recorded by one examiner using a handheld stopwatch to the nearest 0.01 sec. The test was completed 2 times, and the best (shortest) time was used for analysis (Linens et al., 2014; Madsen et al., 2018).
Data analysisDescriptive statistics for demographic data were expressed as mean and standard deviation. The Shapiro–Wilk test was used to check whether the data were normally distributed. The independent sample t-test and paired t-test were applied to compare mean differences in all dependent variables among groups and within groups (pre- and posttest). Cohen effect size (d) and associated 95% confidence intervals were calculated to estimate the magnitude and precision of group differences of each measure. Effect sizes were interpreted as ≥0.80, large; 0.50–0.79, moderate; 0.20–0.49, small; and <0.2, trivial (Cohen, 1992). A P-value of <0.05 was considered significant. All data were analyzed using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA).
RESULTSIn total, 25 athletes with CAI were screened for eligibility to participate in the study. Nine athletes were excluded (six had a history of bilateral ankle instability; two had a knee surgery, and one refused to participate in the study); therefore, a total of 16 athletes with CAI were included in the analysis (Fig. 1). There were no significant differences in participant characteristics at baseline between the groups (Table 2).
Isokinetic strengthFollowing a 4-week intervention, the BFR+R group displayed significant improvements in the mean values of average relative peak torque for the hip extensor (P<0.001; d=0.13, 95% CI, −0.51 to −0.33, trivial), hip abductor (P=0.001; d=0.08; 95% CI, −0.18 to −0.07, very trivial), ankle dorsiflexor (P=0.004; d= 0.05; 95% CI, −0.12 to −0.03, very trivial), ankle plantar flexor (P<0.001; d=0.06; 95% CI, −0.22 to −0.13, very trivial), and ankle evertor (P<0.001; d=0.11; 95% CI, −0.36 to −0.19, trivial) compared with baseline (Fig. 2A–E). However, only significant increases in relative strength were observed for hip extensor (P= 0.023; d=0.07; 95% CI, −0.06 to −0.01, very trivial) and abductor muscles (P=0.011; d=0.06; 95% CI, −0.07 to −0.01, very trivial) for the R group (Fig. 2A, B). In addition, the BFR+R group showed greater improvements in relative strength of ankle plantarflexor (P=0.030; d=1.31; 95% CI, 0.02–0.34, most likely beneficial) and ankle evertor muscles (P=0.020; d=1.36; 95% CI, 0.04–0.52, most likely beneficial) compared with the R group (Fig. 2D–E).
CSA of the muscleAs shown in Table 3, the mean value of the CSA of fibularis longus was significantly increased following an intervention in the BFR+R group compared with baseline (P<0.001) and the R group (P=0.04; d=1.11; 95% CI; 0.03–2.30, most likely beneficial).
Functional performance testAs shown in Table 3, both groups exhibited significant improvements in reaching distance for YBT in the anterior, posteromedial, and posterolateral directions and composite scores over a 4-week intervention (all, P<0.05). There was also a significant improvement in timed performance during SHT in the BFR+R group compared with the R group (P=0.03; d=1.20; 95% CI, −2.41 to −0.05, most likely beneficial).
DISCUSSIONThis study was the first to examine the effectiveness of BFR training combined with traditional rehabilitation program on muscle strength, dynamic balance, and functional performance in athletes with CAI. Herein, we reported the additional effect of BFR+R training on isokinetic strength gains for ankle plantarflexor and evertor, which were associated with great hypertrophy and improvements in functional performance in athletes suffering from CAI compared with the R program alone.
In the present study, a trend toward greater strength gain was observed in all muscles examined, in the BFR+R group compared with the R group. This finding supported our hypothesis, which was consistent with some previous reports. For example, recent systematic reviews and meta-analyses indicated that rehabilitation exercises with BFR are effective in increasing strength in patients with musculoskeletal disorders (i.e., anterior cruciate ligament reconstruction, knee osteoarthritis, and older adults with sarcopenia) compared with traditional rehabilitation alone (Pitsillides et al., 2021). At present, the precise mechanisms underlying BFR-induced strength gain in patients with CAI are unknown. However, most recent studies (Burkhardt et al., 2021; Killinger et al., 2020) have demonstrated considerable muscle activations during acute resistance exercise or dynamic balance exercise with BFR in individuals with CAI compared with controls.
Correspondingly, BFR can potentially lead to a reduction in oxygen delivery to muscle tissue and metabolic by-product clearance. This reduction in blood flow would create hypoxic intramuscular environment, thereby increasing muscle activation via group III and IV muscle afferents (Brandner et al., 2015; Yasuda et al, 2010). In addition, the cortical excitability at the primary control site of lower limb occlusion could spill over to the proximal control site, thereby enabling the recruitment of greater muscle mass. This increased amplitude of motor-evoked potential in the corticospinal region during BFR training may alter motor output to the distal blood flow occlusion area (Madarame et al., 2008; May et al., 2018). Moreover, BFR training can induce high levels of metabolic stress probably because of the accumulation of metabolic waste products (e.g., H+, Pi, and lactate), resulting in the stimulation of anabolic signaling and inflammatory response, thereby leading to enhanced muscle adaptations (Rossi et al., 2018; Takada et al., 2012).
To further determine whether this greater improvement in isokinetic strength after BFR training was primarily because of muscular hypertrophy, the CSA of muscles was measured. However, in the present study, only fibularis longus muscle was selected for CSA measurement because it played a crucial role in dynamic ankle joint stability. Failure to adequately activate this muscle group would impair patient’s ability to control rearfoot supination, thereby allowing the ankle to “giving way” (Palmieri-Smith et al., 2009). In support of this notion, Lobo et al. (2016) reported that the fibularis longus CSA was significantly reduced in individuals suffering from LAS compared with those without LAS. As expected, we found that the BFR+R group elicited a greater hypertrophy of fibularis longus than the traditional rehabilitation alone. Consistent with this finding, Tennent et al. (2017), determined the effects of rehabilitation program with or without BFR in patients with knee arthroscopy and found that the BFR rehabilitation program was more effective in improving strength of the quadriceps muscle, thigh girth, and functional return to activities than a traditional rehabilitation program. Moreover, Ohta et al. (2003) have demonstrated greater quadriceps muscle strength and size in patients after anterior cruciate ligament reconstruction following a 14-week BFR rehabilitation exercise regimen compared with those after traditional rehabilitation. Taken together, these findings suggest that the BFR+R program improves isokinetic strength gains, at least in part, by enhancing muscle hypertrophy.
In addition, impaired dynamic balance has been consistently identified as a major risk factor of CAI (Witchalls et al., 2012). In the present study, the modified star excursion balance test (Y-balance test) was used because it has been proven to be reliable and valid in identifying dynamic balance deficits in those with CAI (Gribble et al., 2012). Unexpectedly, we found no between-group difference in YBT reaching distances in all directions examined following a 4-week intervention. This result differs from the finding of a previous study that showed that dynamic balance exercise with BFR-induced large-to-small increases muscle activation of the ankle muscles and increased postural stability and exertion in individuals with CAI compared with the control (Burkhardt et al., 2021). This discrepancy in results may be because of the differences in methodology (i.e., training protocols and outcome measures) and participant characteristics (athletes vs. sedentary) among studies. In the present study, a supervised rehabilitation exercise (comprising closed chain dynamic balance exercise, squat exercise, double/single-leg heel raises, double/single-limb stance/throwing, and catching on Bosu) was selected because its higher clinical relevance and wide use by clinicians rather than a single, specific rehabilitation exercise as described previously. Clearly, additional studies are warranted to clarify this inconsistency.
Given that individuals with CAI typically report ankle giving way during functional tasks, the SHT was evaluated as a measure of functional deficit related to CAI (Rosen et al., 2019). Our finding that only the BFR+R led to a significant improvement in timed performance during the SHT supports our hypothesis. This finding is consistent with that of a previous study by Yoshida et al. (2018) who reported long performance time (worse) of the involved limb during the SHT and a delayed muscle activity of fibularis longus, tibialis anterior, and gastrocnemius muscles in patients with ankle sprain. The explanation for such improvement is unclear, but it may be related to greater strength gains in the BFR+R versus the R program. Correspondingly, Rosen et al. (2019) reported that the SHT required greater fibularis longus and tibialis anterior muscle activities as the weakness of these muscles have been associated with ankle sprains. Therefore, this BFR+R-induced SHT improvement may be attributed to gaining greater strength in ankle plantarflexor and evertor. Nevertheless, the contribution of other factors (e.g., alterations in neuromuscular control) could not be ruled out, which needs further investigation.
This study has several limitations. First, although the study determines the additional benefits of BFR+R training versus the R alone, the present study did not include an experimental group with BFR only and a control group and crossover design; thus, the synergistic effects were not investigated. Second, considering the relatively small number of participants and their varying sport disciplines used in this study, the result should be interpreted with cautions and not be extrapolated to other athletic populations. Third, the precise mechanisms underlying this BFR+R induced a superior beneficial effect, are not fully elucidated; thus, further studies are required to address this issue. Finally, whether this improvement in outcome measures directly translates into to a lower risk of recurrent injury in athletes with CAI remains unclear.
In conclusion, our results demonstrate that the addition of BFR to traditional rehabilitation program over a 4-week period is more effective in improving strength gain, hypertrophy, and functional performance in athletes with CAI than the traditional rehabilitation alone. This information may help physical therapists and clinicians develop and design a rehabilitation program for athletes with CAI.
ACKNOWLEDGMENTSThe authors would like to thank all athletes who participated in this study. We would also like to thank the Faculty of Sports Science, Chulalongkorn University, for their valuable support. PW was partially supported by a scholarship from the Faculty of Allied Health Sciences, Burapha University, and Graduate School of Chulalongkorn University research grant.
REFERENCESAbe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, KAATSU-walk training. J Appl Physiol. 2006;100:1460–1466.
Boudreau S, Romaniello A, Wang K, Svensson P, Sessle BJ, Arendt-Nielsen L. The effects of intra-oral pain on motor cortex neuroplasticity associated with short-term novel tongue-protrusion training in humans. Pain. 2007;132:169–178.
Brandner CR, Warmington SA, Kidgell DJ. Corticomotor excitability is increased following an acute bout of blood flow restriction resistance exercise. Front Hum Neurosci. 2015;9:652
Burkhardt M, Burkholder E, Goetschius J. Effects of blood flow restriction on muscle activation during dynamic balance exercises in individuals with chronic ankle instability. J Sport Rehabil. 2021;30:870–875.
Delahunt E. Neuromuscular contributions to functional instability of the ankle joint. J Bodyw Mov Ther. 2007;11:203–213.
Emery CA, Meeuwisse MH. The effectiveness of neuromuscular prevention strategy to reduce injuries in youth soccer cluster-randomized controlled trial. Br J Sports Med. 2010;44:555–562.
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–910.
Gerber JP, Williams GN, Scoville CR, Arciero RA, Taylor DC. Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot Ankle Int. 1998;19:653–660.
Gribble PA, Hertel J. Considerations for normalizing measures of the star excursion balance test. Phys Educ Exerc Sci. 2003;7:89–100.
Gribble PA, Hertel J, Plisky P. Using the star excursion balance test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47:339–357.
Gribble PA, Robinson RH. An examination of ankle, knee, and hip torque production in individuals with chronic ankle instability. J Strength Cond Res. 2009;23:395–400.
Hale SA, Hertel J, Olmsted-Kramer LC. The effect of a 4-week comprehensive rehabilitation program on postural control and lower extremity function in individuals with chronic ankle instability. J Orthop Sports Phys Ther. 2007;37:303–311.
Hall EA, Chomistek AK, Kingma JJ, Docherty CL. Balance and strength training protocols to improve chronic ankle instability deficits, Part I: assessing patient-reported outcome measures. J Athl Train. 2018;53:568–577.
Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37:364–375.
Hubbard-Turner T, Turner MJ. Physical activity levels in college students with chronic ankle instability. J Athl Train. 2015;50:742–747.
Jaber H, Lohman E, Alameri M, Bains G, Daher N. The effects of open versus closed kinetic chain exercises on ankle joint function in athletes with chronic ankle instability. J Athl Enhanc. 2018;7:2
Killinger B, Lauver JD, Donovan L, Goetschius J. The effects of blood flow restriction on muscle activation and hypoxia in individuals with chronic ankle instability. J Sport Rehabil. 2020;29:633–639.
Lauver JD, Cayot TE, Rotarius T, Scheuermann BW. The effect of eccentric exercise with blood flow restriction on neuromuscular activation, microvascular oxygenation, and the repeated bout effect. Eur J Appl Physiol. 2017;117:1005–1015.
Linens SW, Ross SE, Arnold BL, Gayle R, Pidcoe P. Postural-stability tests that identify individuals with chronic ankle instability. J Athl Train. 2014;49:15–23.
Lixandrao ME, Ugrinowitsch C, Laurentino G, Libardi CA, Aihara AY, Cardoso FN, Tricoli V, Roschel H. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood flow restriction. Eur J Appl Physiol. 2015;115:2471–2480.
Lobo CC, Morales CR, Sanz DR, Corbalan IS, Marin AG, Lopez DL. Ultrasonography comparison of peroneus muscle cross-sectional qrea in subjects with or without lateral ankle sprains. J Manipulative Physiol Ther. 2016;39:635–644.
Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T, Bemben DA, Bemben MG. Effects of cuff width on arterial occlusion: Implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112:2903–2912.
Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow. Med Sci Sports Exerc. 2008;40:258–263.
Madsen LP, Hall EA, Docherty CL. Assessing outcomes in people with chronic ankle instability: the ability of functional performance tests to measure deficits in physical function and perceived instability. J Orthop Sports Phys Ther. 2018;48:372–380.
May AK, Russell AP, Warmington SA. Lower body blood flow restriction training may induce remote muscle strength adaptations in an active unrestricted arm. Eur J Appl Physiol. 2018;118:617–627.
McKeon PO, Wikstrom EA. The effect of sensory-targeted ankle rehabilitation strategies on single-leg center of pressure elements in those with chronic ankle instability: a randomized clinical trial. J Sci Med Sport. 2018;22:288–293.
Negahban H, Moradi-Bousari A, Naghibi S, Sarrafzadeh J, Shaterzadeh-Yazdi MJ, Goharpey S, Etemadi M, Mazaheri M, Feizi A. The eccentric torque production capacity of the ankle, knee, and hip muscle groups in patients with unilateral chronic ankle instability. Asian J Sports Med. 2013;4:144–152.
Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74:62–68.
Omokawa M, Kinugawa S, Tsutsui H. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol. 2009;106:1119–1124.
Palmieri-Smith RM, Hopkins JT, Brown TN. Peroneal activation deficits in persons with functional ankle instability. Am J Sports Med. 2009;37:982–988.
Patterson SD, Ferguson RA. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J Aging Phys Act. 2011;19:201–213.
Pitsillides A, Stasinopoulos D, Mamais I. Blood flow restriction training in patients with knee osteoarthritis: systematic review of randomized controlled trials. J Bodyw Mov Ther. 2021;27:477–486.
Plisky PJ, Gorman PP, Butler RJ, Kiesel KB, Underwood FB, Elkins B. The reliability of an instrumented device for measuring components of the star excursion balance test. N Am J Sports Phys Ther. 2009;4:92–99.
Rosen AB, Needle AR, Ko J. Ability of functional performance tests to identify individuals with chronic ankle instability: a systematic review with meta-analysis. Clin J Sport Med. 2019;29:509–522.
Rossi FE, Freitas MC, Zanchi NE, Lira FS, Cholewa JM. The role of inflammation and immune cells in blood flow restriction training adaptation: a review. Front Physiol. 2018;9:1376
Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Blood flow restricted exercise for athletes: A review of available evidence. J Sci Med Sport. 2016;19:360–367.
Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Takahashi T, Takada S, Okita K, Suga T, Omokawa M, Morita N, Horiuchi M, Kinugawa S, Tsutsui H. Blood flow restriction exercise in sprinters and endurance runners. Med Sci Sports Exerc. 2012;44:413–419.
Takada S, Okita K, Suga T, Omokawa M, Morita N, Horiuchi M, Kadoguchi T, Takahashi M, Hirabayashi K, Yokota T, Kinugawa S, Tsutsui H. Blood flow restriction exercise in sprinters and endurance runners. Med Sci Sports Exerc. 2012;44:413–419.
Tennent DJ, Hylden CM, Johnson AE, Burns TC, Wilken JM, Owens JG. Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin J Sport Med. 2017;27:245–252.
Valderrabano V, Leumann A, Pagenstert G, Frigg A, Ebtener L, Hintermann B. Chronic ankle instability in sports: a review for sports physicians. Sportverletzung Sportschaden. 2006;20:177–183.
Witchalls J, Blanch P, Waddington G, Adams R. Intrinsic functional deficits associated with increased risk of ankle injuries: a systematic review with meta-analysis. Br J Sports Med. 2012;46:515–523.
Yanagisawa O, Sanomura M. Effects of low-load resistance exercise with blood flow restriction on high-energy phosphate metabolism and oxygenation level in skeletal muscle. Interv Med Appl Sci. 2017;9:67–75.
Fig. 2Average relative peak torque at the angular velocity of 60°/sec of the hip extensor, abductor, ankle dorsiflexor/plantarflexor, and ankle evertor/invertor muscles. BFR, blood flow restriction; R, rehabilitation. *P<0.05, significantly different between pre- and postintervention. #P<0.05, significantly different from the R group. 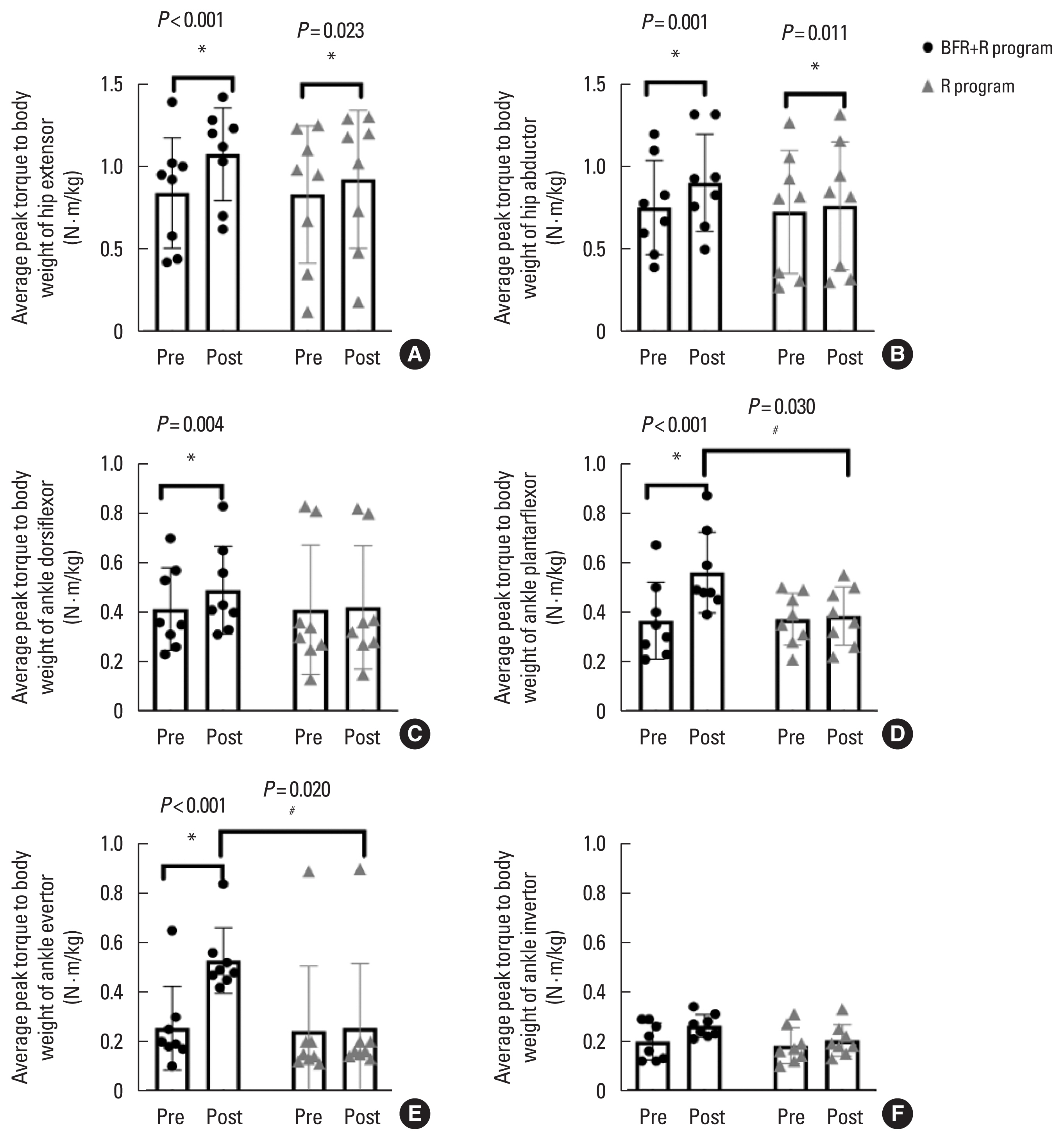
Table 1Outline of the rehabilitation program Table 2Baseline characteristics of the participants. Table 3Mean (standard deviation) values and mean difference (95% CI) of outcome measures during pre- and postintervention among groups
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||