INTRODUCTION
Interest in high-risk pregnancy is increasing due to increased fertility among older women (Gong et al., 2020; Vandekerckhove et al., 2021). One of the problems of maternal aging is that older women lack fertility due to aging, and when they become pregnant, they suffer from diseases such as hypertension and diabetes (Chervenak and Kardon, 1991). As maternal age increases, so does the likelihood of health conditions, gynecological complications, and adverse outcomes (Carolan et al., 2013; Ludford et al., 2012; Vandekerckhove et al., 2021).
Despite this trend, most mothers of older age have good pregnancy outcomes that do not adversely affect health (Carolan, 2013; Walker et al., 2016). This may lead to other women overestimating their fertility, underestimating their risk of future infertility, and underestimating the likelihood of negative consequences if they delay pregnancy (Petersen, 2016). In as much as the trend of delaying pregnancy and childbirth is likely to continue and may accelerate, healthcare providers should monitor (Pawde et al., 2015, Vandekerckhove et al., 2021; Waldenström et al., 2012) and manage maternal health (Carolan, 2013; Wang and Kim, 2015).
Maternal age can also influence child development (Fall et al., 2015), and maternal metabolic status affects offspring physical, neurological development and cognitive function (Berglund et al., 2017; Tuzuka et al., 2009). Increased maternal age is also closely related to decreased fertility and decreased likelihood of giving birth to a healthy child (Wilding, 2014). Research on measures to support maternal aging in pregnancy is centered on high-risk mothers, and little attention is paid to the outcome of the offspring.
Therefore, this study was conducted to find out whether active exercise training for children of old mothers is an effective countermeasure against the adverse effects on cognitive function. The effect of exercise was studied using mice comparing the spatial learning memory of offspring born to older mothers to offspring born to young mothers.
MATERIALS AND METHODS
Animals
The offspring of mice (4 weeks) were assigned into four groups after delivery (n=10 in each group): offspring of young mothers (control) group, offspring of young mothers with exercise group, offspring of old mothers group, and offspring of old mothers with exercise group. All animal experiments were performed in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Science. The study protocol was approved by the Kyung Hee University Institutional Animal Care and Use Committee (approval number: KHUASP [SE]-18-132). 5-Bromo-2′-deoxyuridine (BrdU) (Sigma, St. Louis, MO, USA) was administered intraperitoneally at 100 mg/kg/day to observe neurogenesis of offspring.
Exercise protocol
The exercise groups performed running on a treadmill once daily for 6 days per week for 4 consecutive weeks. A load of exercise consisted of running at the initial speed of 3 m/min for the first 5 min, 5 m/min for the next 5 min, and 10 m/min for the last 30 min with the 0° inclination.
Preparation of tissue samples
Mice were euthanized immediately after the behavior test. To prepare brain slices, the animals were fully anesthetized with ethyl ether, perfused transcardially with 50 mM phosphate-buffered saline (PBS), and then fixed with a freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred to a 30% sucrose solution for cryoprotection. Coronal sections with 40-μm thickness were created using a freezing microtome (Leica, Nussloch, Germany). From each group of 10 animals, 5 were used for immunofluorescence and five for western blotting.
Morris water maze
Morris water maze test was conducted to measure spatial learning memory. The test animals were acclimated by swimming freely for 60 sec in a pool without a platform one day before the start of training. The educational training was performed 3 times a day for 5 days with a platform. If the animal was unable to find the location of the platform within 60 sec, the experimenter guided the animal to the platform. Then, the animal remained on the platform for 30 sec. A probe trial was conducted 24 hr after the training session. Free swimming for 60 sec without a platform was automatically evaluated by video tracking to determine whether the memory of the previous platform was retained or not.
Immunofluorescence
Neuronal nuclei (NeuN)/BrdU-positive cells in the dentate gyris were evaluated by immunofluorescence. The brain sections were permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, incubated in 50% formamide-2×standard saline citrate at 65°C for 2 hr, denatured in 2 N HCl at 37°C for 30 min, and then rinsed twice in 100 mM sodium borate (pH, 8.5). The sections were incubated overnight with rat anti-BrdU antibody (1:200; Abcam, Cambridge, UK) and mouse anti-NeuN antibody (1:200; Millipore, Temecula, CA, USA). The brain sections were then washed in PBS and incubated with appropriate secondary antibodies for 1 hr. The secondary antibodies were anti-mouse IgG Alexa Fluor-488 and anti-rat IgG Alexa Fluor-560.
Quantification of histology
For quantification of immunofluorescence for the hippocampus, slices spanning −3.00 to −3.72 mm from the bregma were obtained from each brain. Images were captured using a BX51TF microscope (Olympus, Tokyo, Japan) for immunohistochemistry and an FV3000 confocal microscope (Olympus) for a subsequent Z-section (1 μm) analysis for immunofluorescence. For positive cell counts, images were photographed and quantified dentate gyrus fields that were randomly chosen from each brain section. A total of two fields were manually analyzed per mouse by one blind experimenter. From each group of 10 animals, 5 were used for immunofluorescence and two sections from each group were analyzed, resulting in a total of 10 slices. Quantification of immunofluorescence were calculated by dividing the number of positive cells by the area of the dentate gyrus.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
To visualize DNA fragmentation, TUNEL staining was performed using an In Situ Cell Death Detection Kit (Roche Diagnostics, Risch-Rotkreuz, Switzerland) according to the manufacturer’s protocol. Sections were postfixed in ethanol-acetic acid (2:1), rinsed, incubated with proteinase K (100 mg/mL), and then rinsed again. Next, they were incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% DAB, counterstained with cresyl violet, and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature. A coverslip was added using Permount mounting medium.
Western blotting
Hippocampal tissues were homogenized on ice and lysed in lysis buffer. Antibodies for mouse β-actin (1:1,000), mouse Bcl-2 related X protein (Bax) (1:1,000), rabbit B-cell lymphoma 2 (Bcl-2) (1:1,000), rabbit brain-derived neurotrophic factor (BDNF; 1:1,000), rabbit postsynaptic density protein 95 (PSD-95, 1:1,000) were used as the primary antibodies. Horseradish peroxidase-conjugated secondary anti-mouse antibodies were used for β-actin and Bcl-2. Horseradish peroxidase-conjugated secondary anti-rabbit antibodies were used for Bax, BDNF, and PSD-95.
Statistical analyses
Data quantification were performed using Image-Pro Plus (Media Cyberbetics Inc., Rockville, MD, USA) attached to a light microscope (Olympus). The data were analyzed using a one-way analysis of variance, followed by Tukey post hoc tests. All values are expressed as mean±standard error of the mean, and P values of <0.05 were considered significant.
RESULTS
Effect of exercise on spatial learning memory in offspring
Morris water maze test assessed for spatial learning memory (Fig. 1). Spatial learning memory was significantly reduced only in the offspring of old mothers on days 3, 4, and 5. When compared with the control group, the offspring of old mothers showed significantly reduced spatial learning memory (P=0.037). However, the offspring of old mothers with exercise showed increased spatial learning memory (P=0.012).
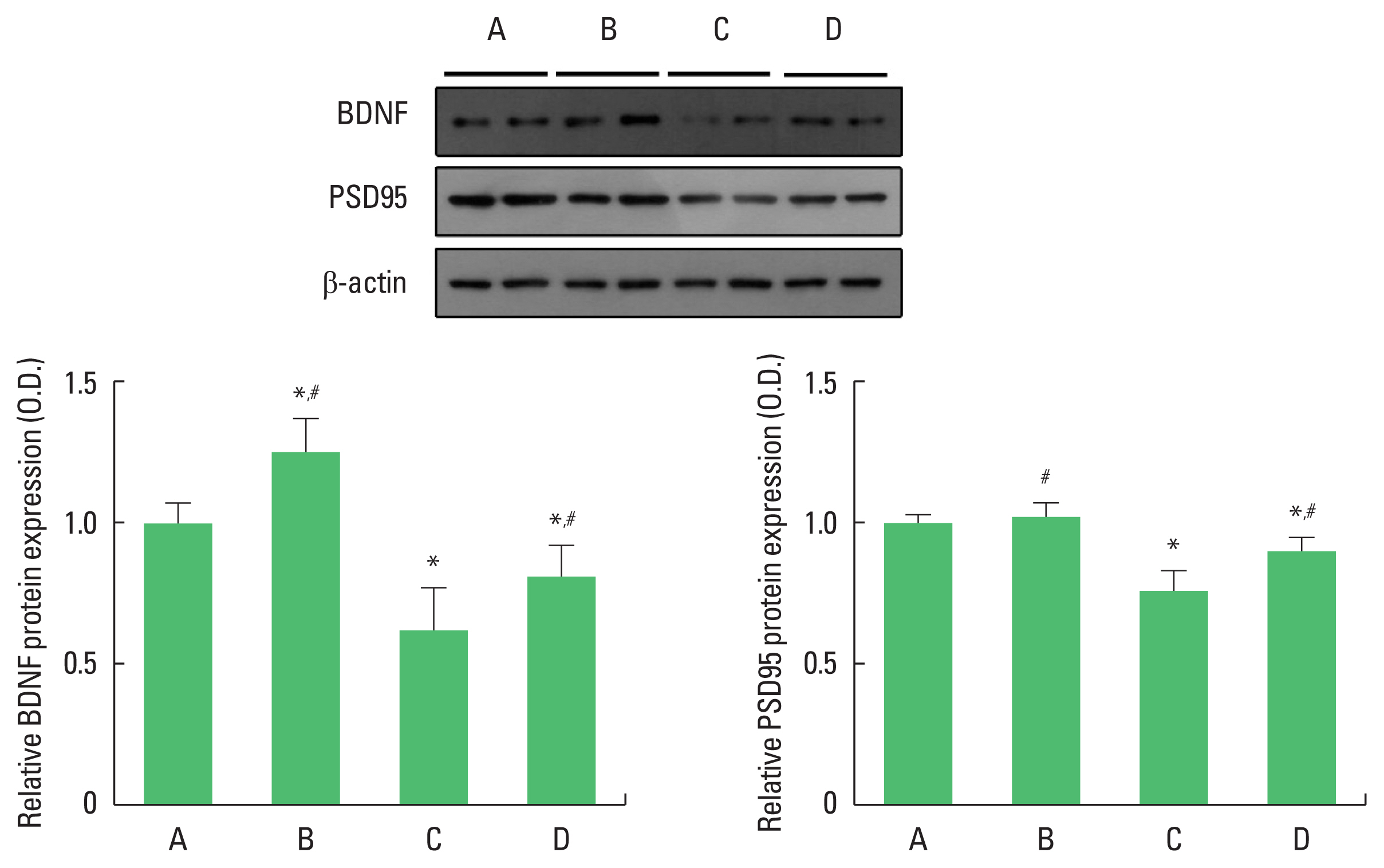
Effect of exercise on hippocampal BDNF and PSD-95 levels in offspring
We performed western blotting to determine hippocampal BDNF and PSD-95 protein levels (Fig. 2). For between-group comparisons, the value of the control group was set to 1 and relative values were assigned to the other groups. Compared with the control group, the offspring of old mothers showed significantly decreased BDNF (P<0.001) and PSD-95 (P<0.001) protein levels. In contrast, the offspring of old mothers with exercise showed significantly increased BDNF (P=0.017) and PSD-95 (P=0.035) levels.
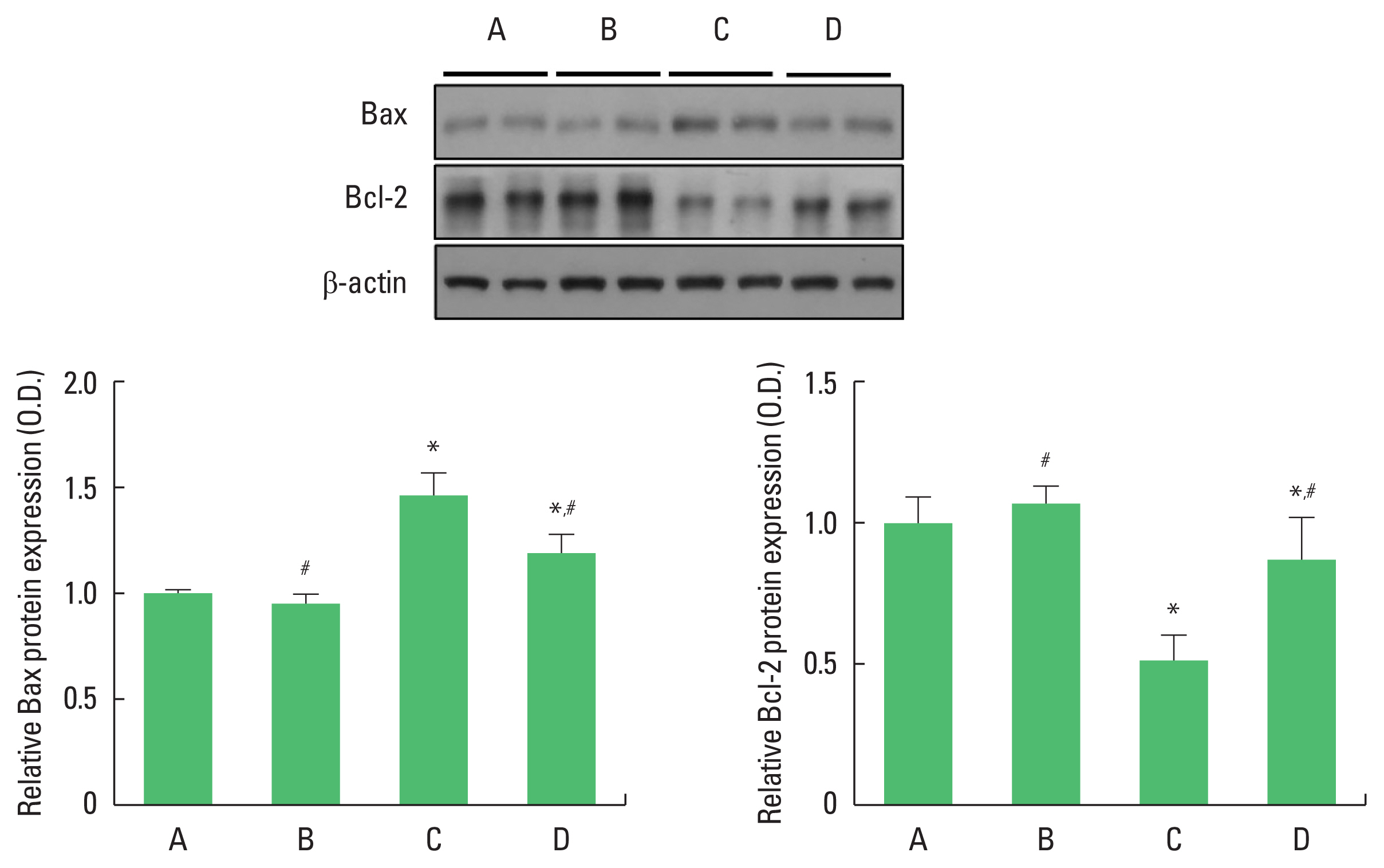
Effect of exercise on hippocampal Bax and Bcl-2 expressions in offspring
We analyzed Bax and Bcl-2 protein expressions (Fig. 3). The value of the control group was set to 1 and the other groups were assigned relative values. Compared with the control group, the offspring of old mothers showed increased Bax and decreased Bcl-2 protein expressions (P<0.001). In contrast, there was a significant decrease in Bax and increase Bcl-2 protein expressions (P< 0.001) in the offspring of old mothers with exercise.
Effect of exercise on hippocampal cell death in offspring
To examine hippocampal cell death, we analyzed TUNEL-positive cells (Fig. 4). Compared with the control group, the offspring of old mothers showed significantly increased TUNEL-positive cells in the hippocampus (P<0.001). In contrast, the offspring of old mothers with exercise showed decreased TUNEL-positive cells (P=0.022).
Effect of exercise on hippocampal neurogenesis in offspring
To examine hippocampal neurogenesis, we analyzed NeuN/BrdU-positive cells (Fig. 5). Compared with the control group, the offspring of old mothers showed significantly decreased NeuN/BrdU-positive cells (P=0.019) in the hippocampus. In contrast, NeuN/BrdU-positive cells were significantly increased in the offspring of old mothers with exercise (P=0.021).
DISCUSSION
As women’s socioeconomic status rises, the trend of increasing fertility in the old is expected to continue. This can have social and public health impacts and increase demand for healthcare (Biro et al., 2012). The increase in maternal age due to delayed childbirth has negative effects on the child, as seen in negative pregnancy outcomes (Sandin et al., 2012), but can be prevented through early detection, treatment and management (Odame Anto et al., 2018). It also suggests that active health management can alleviate the fear of these side effects. Our study conducted exercise training as an experimental treatment for offspring of rodents of higher maternal age.
In this study, spatial learning memory was impaired and hippocampal BDNF production was decreased in offspring of old mothers. New cell proliferation and synaptic plasticity were decreased and apoptotic cell death was increased in offspring of old mothers.
Maternal age and maternal metabolic status affect offspring cognitive function (Berglund et al., 2017; Tuzuka et al., 2009; Wilding, 2014). A higher maternal age is associated with an intelligence quotient in the child (Myrskylä et al., 2013), and newborns of mothers with diabetes have increased insulin and oxygen consumption, which influences the child’s behavioral and intellectual development (Eidelman and Samueloff, 2002; Rizzo et al., 1991).
The present study showed that exercise for offspring of old mothers showed improved performance in the Morris water maze test. This means that exercise improved spatial learning memory in the offspring of old mothers. Exercise increased hippocampal neurogenesis, synaptic plasticity, and BDNF expression in the offspring of old mothers. Exercise also decreased apoptotic cell death in the offspring of old mothers.
Many studies suggest that physical exercise has a positive effect on cognitive function (Falck et al., 2019; Jia et al., 2019; Ptomey et al., 2018). Physical activity also significantly influences children’s achievement and cognitive function (García-Hermoso et al., 2021; Meli et al., 2021; Visier-Alfonso et al., 2021). Low-intensity treadmill exercise in young rats affects cognitive function by increasing neurogenesis and BDNF level in the dentate gyrus (Lou et al., 2008). Exercise is recommended for children with conditions such as diabetes (Naylor et al., 2016; Quirk et al., 2014), and moderate-intensity chronic exercise improved overall executive function in children and adolescents with attention deficit/hyperactivity disorder (Liang et al., 2021).
Exercise can be an effective countermeasure against memory decline in the offspring of old mothers. In other words, exercise can relieve the psychological and physiological burden of old women considering childbirth and improve the aftereffects of babies due to old age.