INTRODUCTION
Obesity leads to the accumulation of body fat that poses health risks. Diet and exercise have been suggested as methods of treating obesity. Regular exercise and an appropriate diet are important to treat obesity (Jakicic et al., 2001). Imbalance in the gut microbiota can lead to diseases and is closely related to obesity. When the gut microbiota of obese mice was transplanted into germ-free mice, body weight increased and recipients developed metabolic disorders (Ley et al., 2006). In addition, it was reported that there is a difference in the diversity of the intestinal microflora when comparing the gut microbiome of normal and obese people (Crovesy et al., 2020).
The gut microbiome interacts with the nervous system via the gut-brain axis, a communication channel between the central nervous system and the enteric nervous system (Carabotti et al., 2015). The microbiome has the potential to affect brain and mental health (Rogers et al., 2016). In depression, Bacteroidetes, Protobacteria, and Actinobacteria increased and Firmicutes decreased (Jiang et al., 2018; Winter et al., 2018). These findings suggest that change in the gut environment may affect brain function and psychiatric disorders such as depression and anxiety.
Treatment of obesity includes dietary changes, increased physical activity, and medication. Aerobic exercise is recommended for body fat reduction (Cox et al., 2004), and low-intensity aerobic exercise is known to be the most effective treatment for obesity (Donnelly et al., 2004). Cook et al. (2016) reported that physical activity improved gut immune function by altering the gut environment. Exercise is known to modulate glucose intolerance, fat removal and insulin sensitivity (Conn et al., 2014) and to lower the risk of type 2 diabetes and other metabolic disorders (Rattanavichit et al., 2018). Exercise upregulates activity of insulin signaling pathway and increases glucose uptake by skeletal muscle (Hawley and Lessard, 2008). Exercise improved the diversity and composition of the gut microbiota in obese mouse model (Petriz et al., 2014). Voluntary exercise increased the diversity of the gut microbiota, and this improvement in gut microbiota effectively modulated glucose homeostasis (Evans et al., 2014). The possibility that the composition of the intestinal microflora changes with exercise has been suggested, but clear evidence is still lacking.
In this experiment, we studied the effects of treadmill exercise and diet on brain function, insulin signaling pathway, and intestinal microbiome in obesity-induced mice. A more effective method for obesity during exercise or diet was tried to find.
MATERIALS AND METHODS
Experiment animals and treatments
The male BL6 mice (8-month-old) were housed under controlled temperature (24°C±2°C) and lighting (07:00 a.m. to 19:00 p.m.) conditions. This experimental protocol was approved by the Kyung Hee University Institutional Animal Care and Use Committee (approval number KHUASP [SE]-20-15). The animals were randomly divided into five groups (n=10 per group): control group, exercise group, obesity group, obesity with exercise group, and obesity with diet group.
Before the start of this experiment, obesity groups made obese mice by administering a high-fat diet containing 60% fat for 12 weeks. In the obesity with exercise group, after a high-fat diet for 12 weeks, exercise was performed with high-fat diet for 8 weeks. In the obesity with diet group, a high-fat diet for 12 weeks followed by a normal diet for 8 weeks.
Treadmill exercise protocol
The treadmill exercise was performed once a day, 6 days per week for 8 consecutive weeks. The exercise groups performed 5 min of warm-up at a 0° inclination at 3 m/min, 40 min of the main exercise at 10 m/min, and 5 min of cool down at 3 m/min for the first 2 weeks. Following this period, animals performed 40 min of the main exercise at 10 m/min during weeks 1–2, 40 min of the main exercise at 13 m/min during weeks 3–4, 50 min of the main exercise at 13 m/min during weeks 5–8.
Open field test
As previously described method (Park et al., 2020b), mice activity was determined by open field test. The animal was randomly assigned to an order of testing and placed in a white square open field arena (40×40 cm) made of wood, enclosed by 30-cm-high walls and exposed to strong illumination (200 lux). The arena was divided into 25 squares (20 cm×20 cm), consisting of nine central and 16 peripheral squares. The animals were placed in the center of the arena and were allowed to explore the environment for 60 sec. After that time, the number of squares crossed was recorded for 5 min.
Elevated plus maze test
As previously described method (Park et al., 2020a), anxiety behavior was determined by elevated plus maze test. The elevated plus maze test apparatus consisted of two open arms, crossed at right two opposed arms of the same size. The junction area of central measured platform set up 65 cm above the floor. The mice were placed on the center platform facing a closed arm and were allowed to explore the maze freely for 5 min. Entry into an arm was defined as entry of all four paws into the arm. Entries and time in the open arms were measured.
Tissue preparation
The animals were sacrificed immediately after determination of elevated plus maze test. To prepare the brain slices, the mice were fully anesthetized by intraperitoneal injection of Zoletil 50 (10 mg/kg, Vibac Laboratories, Carros, France). The mice were transcardially perfused with 50 mM phosphate-buffered saline and then fixed with freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections with thicknesses of 40 μm were made using a freezing microtome (Leica, Nussloch, Germany).
Western blotting for insulin signaling
As previously described method (Park et al., 2019), the brain hippocampus was homogenized on ice and lysed in a lysis buffer. Protein of 40 μg was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane, which was incubated with mouse β-actin antibody (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit phosphorylated phosphoinositide 3-kinase (p-PI3K) antibody (1:1,000; Cell Signaling Technology, Danvers, MS, USA), rabbit total PI3K antibody, rabbit phosphorylated protein kinase B (p-AKT) (1:1,000; Cell Signaling Technology), total AKT (t-AKT) (1:1,000; Cell Signaling Technology). Horseradish peroxidase-conjugated anti-mouse for β-actin and anti-rabbit for p-PI3K, t-PI3K, p-AKT, t-AKT were used as secondary antibodies.
Immunohistochemistry for tryptophan hydroxylase in dorsal raphe
As previously described method (Park et al., 2020a), immunohistochemistry for tryptophan hydroxylase (TPH) in the dorsal raphe were performed. The sections were then incubated in 1% hydrogen peroxide (H2O2) for 25 min. The sections were incubated overnight with rabbit anti-TPH antibody (1:200; Oncogene Research Product). The sections were incubated for 90 min with biotinylated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA), and they were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 90 min. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% 3,3′-diaminobenzidine and 0.01% H2O2 in 50 mM Tris-buffer (pH, 7.6) for approximately 3 min. The sections were finally mounted on gelatin-coated glass slides.
Collection of fecal samples, and sequencing and metagenomic analysis
Fecal samples collected as previously described (Jang et al., 2020; Kim et al., 2021) were placed in sterile test tubes and stored at −80°C. Total DNA in feces was extracted from feces at 200 mg per sample using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Sequencing library protocol for amplifying V3 and V4 regions, 2 ng of input gDNA was prepared using 5× reaction buffer, 1 mM dNTP mixture, 500 nM each of universal F/R PCR primers and Herculase II fusion DNA polymerase (Agilent Technologies, Santa Clara, CA, USA). The cycle conditions for the primary PCR were 25 cycles of 3 min at 95°C, 30 sec at 95°C, 30 sec at 55°C, and 30 sec at 72°C for thermal activation followed by a final 5 min extension at 72°C.
The primary PCR product was purified with AMPure beads (Agencourt Bioscience, Beverly, MA, USA). After purification, 2 μL of the primary PCR product was PCR amplified using NexteraXT Indexed Primer to construct a final library containing the index. The cycle conditions of the second PCR were the same as those of the first PCR except for 10 cycles. The PCR product was purified with AMPure beads. The final purified product is then quantified using qPCR according to the qPCR quantification protocol guide (KAPA Library Quantification Kit for Illumina Sequencing Platforms) and qualified using a TapeStation D1000 ScreenTape (Agilent Technologies, Waldbronn, Germany). and sequenced using the MiSeq platform (Illumina, San Diego, CA, USA). The barcoded 16S rRNA gene amplicons were sequenced using the Illumina MiSeq platform from Macrogen Inc. (Seoul, Korea). DNA representing the fecal microbiota extracted from feces for whole metagenomic shotgun sequencing was sequenced using paired-end shotgun sequencing using the Illumina Hi-Seq 2000 platform from Macrogen Inc.
Data analysis
For confirming the expressions of insulin signaling proteins, the detected bands were calculated densitometrically using Molecular Analyst ver. 1.4.1 (Bio-Rad, Hercules, CA, USA). The numbers of TPH-positive cells in the dorsal raphe were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan). The data were analyzed with one-way analysis of variance and then Tukey post hoc tests. All values are expressed as the mean ±standard error of the mean, and P value <0.05 was considered significant.
RESULTS
The effects of exercise and diet on depression and anxiety
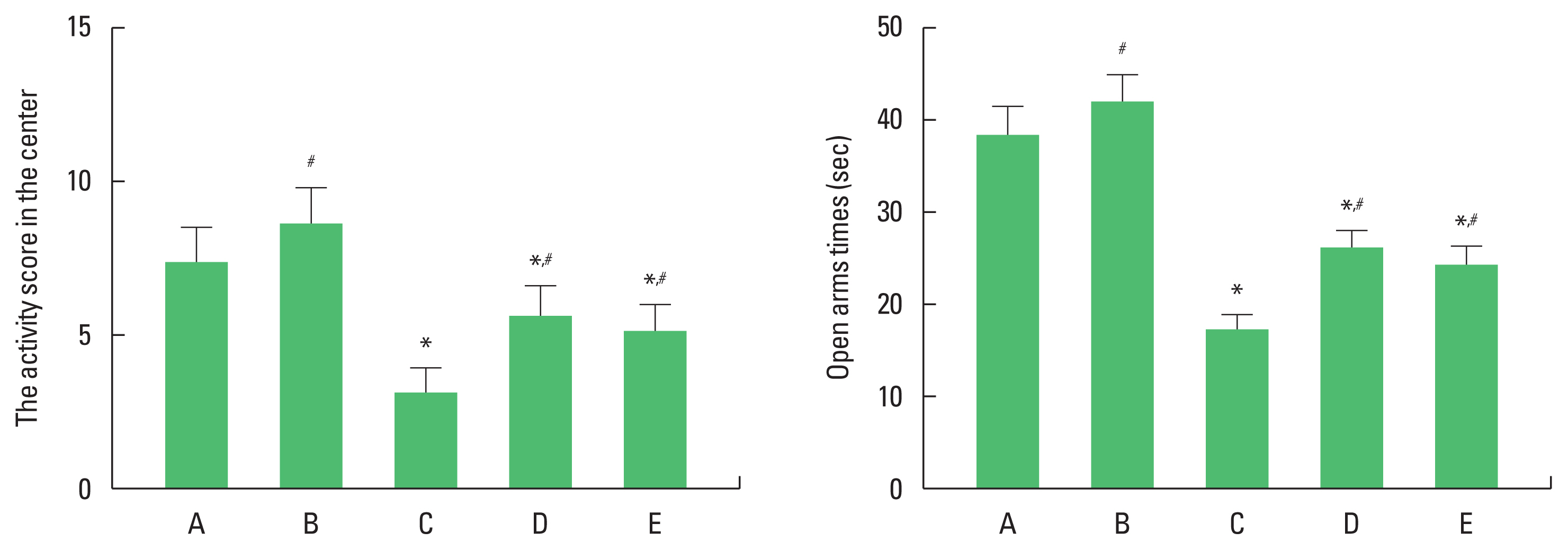
Activity score in the center of the open field test and open arms times of elevated plus maze test were decreased in the obesity group. Exercise and diet increased activity score in the center of the open field test and open arms times of elevated plus maze test in the obesity group (Fig. 1). There was no statistical significance in depression and anxiety status between obesity with exercise group and obesity with diet group. These results demonstrated that obesity showed depression and anxiety status, in contrast, exercise and diet suppressed depression and anxiety status.
The effects of exercise and diet on insulin signaling molecules in the hippocampus
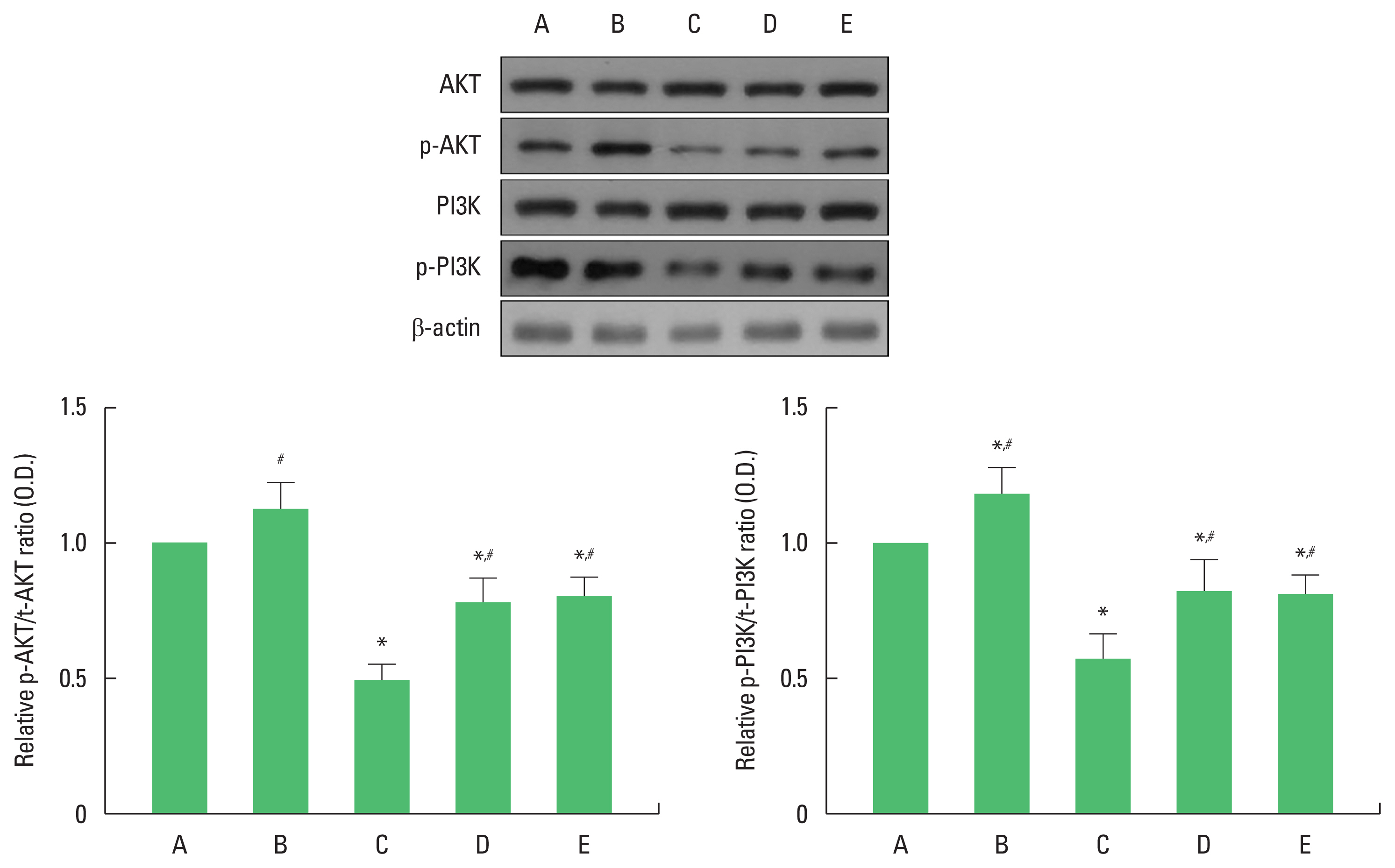
To examine changes of insulin signaling molecules in the hippocampus, the control group was set at 1.00. Ratio of p-AKT/t-AKT ratio and p-PI3K/t-PI3K were decreased in the obesity group. Exercise and diet increased p-AKT/t-AKT ratio and p-PI3K/t-PI3K ratio in obesity mice (Fig. 2). There was no statistical significance in ratio of p-AKT/t-AKT and p-PI3K/t-PI3K between obesity with exercise group and obesity with diet group. These results demonstrated that obesity suppressed insulin signaling pathway, in contrast, exercise and diet activated insulin signaling pathway.
The effects of exercise and diet on TPH expression in the dorsal raphe
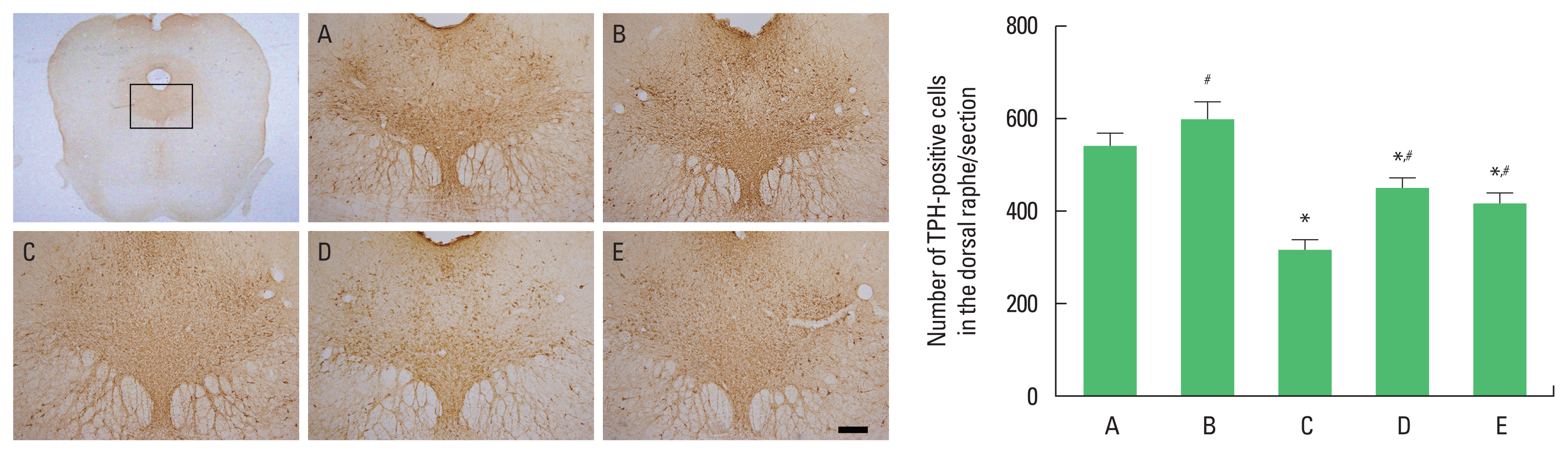
The number of TPH-positive cells in the dorsal raphe was decreased in the obesity group. Exercise and diet increased the number of TPH-positive cells in obesity mice (Fig. 3). There was no statistical significance in TPH expression between obesity with exercise group and obesity with diet group. These results demonstrated that obesity inhibited serotonin synthesis, in contrast, exercise and diet increased serotonin synthesis.
The effects of exercise and diet on microbiome in the gut microbiota
The diversity of intestinal microbiota was shown in Fig 4. The % of Bactroidetes and counts of Lactobacillus acidophilus in the microbiome were decreased in the obesity group. Exercise and diet increased % of Bactroidetes and counts of L. acidophilus in obesity mice. The % of Firmicutes in the microbiome was increased in the obesity group. Exercise and diet decreased the % of Firmicutes in obesity mice. There was no statistical significance in % of Bactroidetes, counts of L. acidophilus, % of Firmicutes between obesity with exercise group and obesity with diet group. These results demonstrated that obesity suppressed Bactroidetes and L. acidophilus and enhanced Firmicutes, in contrast, exercise and diet increased Bactroidetes and L. acidophilus and inhibited Firmicutes.
DISCUSSION
In this study, exercise and diet were suggested as the most effective ways to treat obesity, and the effects were almost similar. Exercise is recommended as an effective way to reduce neuroplasticity in the brain and levels of depression and anxiety (De Moor et al., 2006). Aerobic exercise has been shown to prevent depression (Teychenne et al., 2008).
Insulin dysregulation is associated with diabetes, obesity, and cardiovascular diseases (Craft, 2005). Abnormality in the insulin signaling system affects a variety of neurodegenerative diseases and brain function (van der Heide et al., 2006). Insulin induces depression primarily through activation of the cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) pathway (Xia et al., 2019). It was also reported that insulin function is reduced by PI3K inhibitors (Detka et al., 2019). As such, the insulin signaling system goes beyond simple blood sugar control and affects brain function, causing anxiety and depression. In this study, obesity showed depression and anxiety status and suppressed ratio of p-AKT/t-AKT and p-PI3K/t-PI3K. Exercise and diet suppressed depression and anxiety status via enhancing ratio of p-AKT/t-AKT and p-PI3K/t-PI3K.
TPH is an enzyme involved in the synthesis of the neurotransmitter serotonin. Serotonin plays a role in regulating mood, depression, and memory, and is affected by a variety of factors such as anxiety and stress (Michelsen et al., 2008). Aerobic exercise improved depression symptoms by increasing serotonin synthesis through activation of TPH and serotonin 1A receptor (Kim et al., 2015). Exercise has a direct effect on increasing the concentration of tryptophan in the blood that crosses the blood-brain barrier to the central nervous system (Steiner, 2011). In this study, exercise and diet increased the number of TPH-positive cells.
Maes et al. (2008) reported that when the diversity of the gut microbiota decreased, the incidence of depressive symptoms increased, and this change in the gut microbiota was directly affected by the diet. Carbohydrate intake and metabolism can accelerate change in the intestinal environment, which ultimately lead to depressive symptoms (Haghikia et al., 2015). In obesity model, the level of Firmicutes as well as lipid absorption were higher, which made the animal more susceptible to obesity (Magne et al., 2020). Exercise modulates obesity progression by improving the Firmicutes/Bactroidetes ratio, which could potentially contribute to weight loss and gastrointestinal disorders (Aragón-Vela et al., 2021). In this study, obesity inhibited Bactroidetes and L. acidophilus and increased Firmicutes. Exercise and diet increased Bactroidetes and L. acidophilus and inhibited Firmicutes.
Exercise and diet improved depression and anxiety status by increasing the insulin signaling pathway and promoting serotonin production. These effects of exercise and diet were almost similar. In addition, exercise and diet regulated the composition of gut microbiota.