INTRODUCTION
One of the serious consequences of aging involves a decline in the function of the brain. The aging brain is associated with various neurodegenerative diseases such as Alzheimer disease, which mainly impacts cognitive function of the elderly, and Parkinson disease, which mainly impacts their motor function. Even if an individual ages without a devastating neurodegenerative disease, it is inevitable that he will experience a gradual decline in both cognitive and motor functions of the brain. Nevertheless, slowing that process may still be possible through physical activity (PA). Indeed, PA is known to slow, or even reverse, the aging processes in many body systems, such as cardiovascular, metabolic, endocrine and skeletal functions (Nelson et al., 2007, Nied and Franklin, 2002). It has also been shown that PA can improve cognitive function in the elderly (Colcombe et al., 2006; Kramer and Colcombe, 2018; Kramer and Erickson, 2007).
Potential benefits of PA for maintaining motor function of the brain have also been suggested. For example, Wang et al. (2016) conducted a study in which they investigated interlimb transfer of visuomotor adaptation, which is a type of motor learning that is studied frequently in systems neuroscience, in healthy older adults. In that study, the participants were divided into two groups (physically active vs. sedentary) and adapted to a novel visuomotor rotation condition. The results showed that in physically active older adults, transfer of visuomotor adaptation occurred asymmetrically, that is, from the nondominant arm to the dominant arm, in terms of directional control during targeted reaching movements, but not from the dominant to the nondominant arm. This result is consistent with the findings observed in healthy young adults, who also showed the same asymmetrical transfer (Sainburg and Wang, 2002). The authors argued that interlimb transfer occurred asymmetrically because of the lateralized motor function of each brain hemisphere. That is, the dominant hemisphere/limb system is specialized for controlling directional features of reaching movements (Sainburg and Wang, 2002; Wang and Sainburg, 2006). Thus, when an individual adapts to a visuomotor rotation condition with the nondominant arm first, then with the dominant arm, the dominant hemisphere/limb system can access and utilize directional information obtained during prior adaptation with the nondominant arm, thus resulting in interlimb transfer. However, when he adapts to a rotation condition with the dominant arm first, then with the nondominant arm, the nondominant system cannot access directional information obtained during prior adaptation with the dominant arm (thus no transfer) because controlling the directional features is not its specialty. The fact that asymmetrical transfer was observed in both young adults and physically active older adults indicates that physically active older adults have intact function of the dominant hemisphere in terms of directional control. In contrast, transfer occurred symmetrically in sedentary older adults, indicating diminished function of the dominant hemisphere in older adults who live a sedentary lifestyle.
This argument is in line with the findings that performance of a certain cognitive task, which typically involves a single brain hemisphere in young adults, requires the recruitment of additional brain areas spread across both brain hemispheres in older adults (Bergerbest et al., 2009; Cabeza et al., 2004). These findings suggest a decline in the function of a single brain hemisphere involved in the given cognitive task with aging, which led to the HAROLD model (hemispheric asymmetry reduction in older adults). This model has been supported by a number of studies that used different types of cognitive tasks such as episodic, semantic and working memory and visual perception tasks (Cabeza et al., 2004; Dolcos et al., 2002; Rypma et al., 2001). The findings reported by Wang et al. (2011, 2016) support and extend the HAROLD model by demonstrating that hemispheric asymmetry reduction in older adults is also observed in the motor domains, and that it may depend on one’s lifestyle.
Among numerous types of PAs, walking is one of the most popular activities performed by many people because it is easy to perform and cost-effective. One’s walking ability is known to be related directly to the health of many body systems including the central nervous system and the perceptual system (Ferrucci et al., 2000). It is also related to improved cognitive and executive function (Prohaska et al., 2009; Weuve et al., 2004; Yogev-Seligmann et al., 2008). Given that, Kang et al. (2021) recently developed an exercise program called the cognitive walking program (CWP), which was designed to improve cognitive function of the elderly by stimulating their memory, attention, visual perception and walking ability while performing various stepping patterns that require specific motions of the upper and lower extremities. The effect of the CWP on improving cognitive function in healthy older adults has been demonstrated in a recent study by Kang et al. (2021). However, it remains unknown whether the CWP is also beneficial for improving motor function of the brain. In the current study, thus, we investigated the effects of the CWP on motor function of the brain by examining changes in the pattern of interlimb transfer following visuomotor adaptation in physically active and sedentary older adults.
MATERIALS AND METHODS
Subjects
Subjects were recruited by distributing flyers and also by word of mouth through two community healthcare centers in Seoul, Korea in 2018 and 2019. (Participants who joined the program later in 2019 were unable to complete it due to the coronavirus disease 2019 pandemic.) The inclusion criteria were as follows: (a) at least 65 years old, (b) right-handed, and (c) normal cognitive function as assessed through Korean Mini-Mental State Examination (K-MMSE). The exclusion criteria were as follows: (a) a recent history of severe cardiovascular disease, (b) neurological disease or peripheral disorder affecting movement of their arms, (c) significant orthopedic conditions limiting mobility, (d) visual impairment, (e) probable dementia as assessed by K-MMSE (score <24), and (f) any other factors that could potentially limit the ability to fully participate in this study (e.g., musculoskeletal problems, severe depression, plans to leave out of town for more than 4 days in a row during the participation period, etc.).
All subjects were explained about the study at the time of recruitment and signed a consent form prior to their participation in the study. This consent form was approved by the Institutional Review Board of Sangmyung University (# BE2017-26). After recruitment, participants were classified as active or sedentary according to the Stanford Brief Active Survey (SBAS) (Taylor-Pillae et al., 2010). Those who indicated to engage in vigorous intensity PA three or more days per week (e.g., jogging, running, bicycling or swimming for 30 min or more) in the SBAS were classified as active. Those who indicated low intensity PA 2 or fewer days per week (ranging from no PA to slow walking or light chores) were classified as inactive. Active participants were assigned to the CWP-active group (CWP-A), and sedentary participants were assigned to the CWP-sedentary group (CWP-S). A control group was included in the study, in which sedentary participants were assigned to a normal walking program (NW-S), which involved typical walking outdoor. At baseline (T0) and 3 months (T1) after the beginning of the exercise program, there were a total of 75 participants (CWP-A=31, CWP-S=29, NW-S=15). Among them, 52 participants completed the program (CWP-A=20, CWP-S=21, NW-S=11), whose data were used in the final analysis. The average age of the three groups was 72.40±3.90, 73.29+4.80, and 74.45±4.47 years for the CWP-A, CWP-S, and NW-S group, respectively, which were not significantly different from each other (P>0.05).
Exercise programs
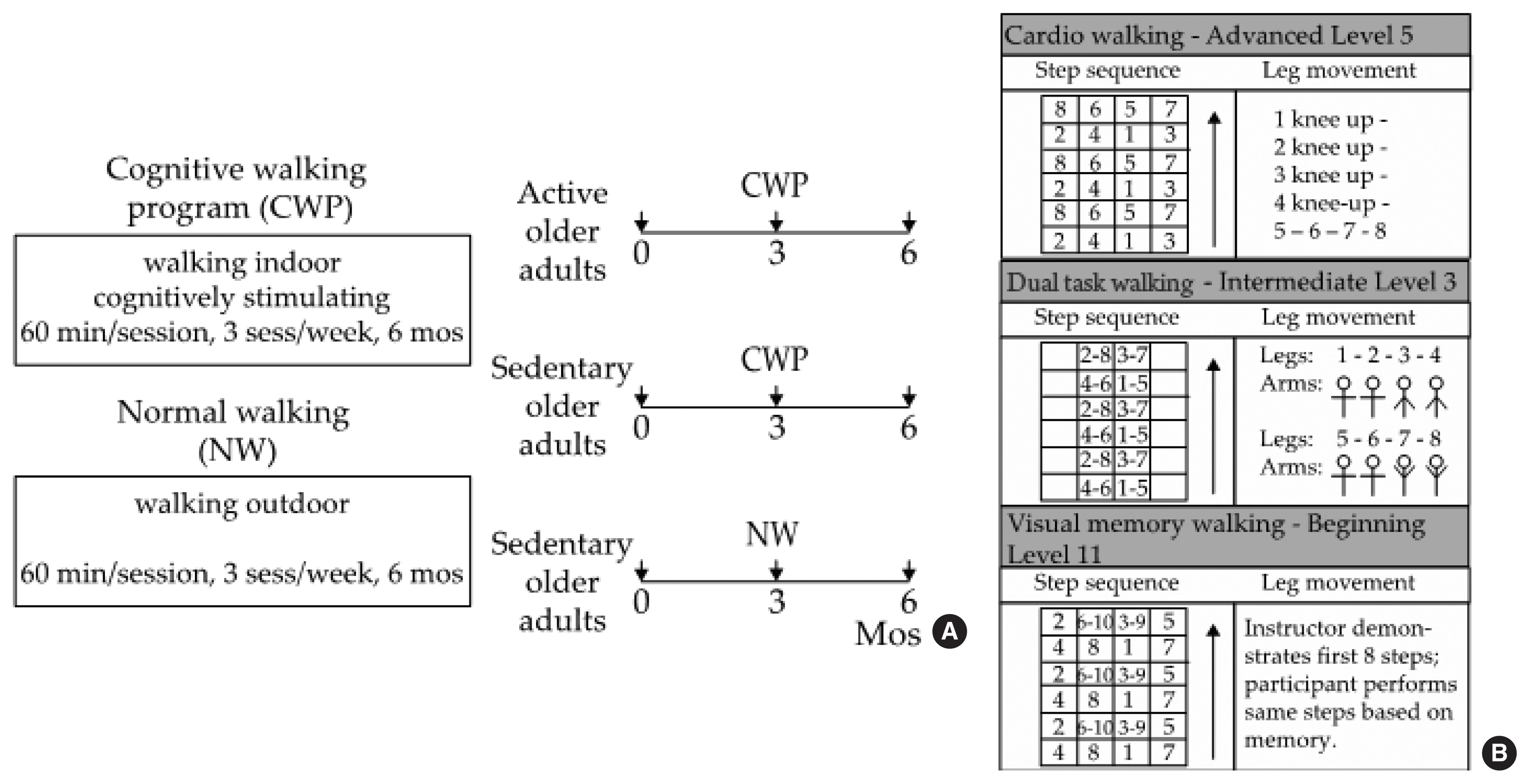
The subjects who were assigned to the CWP performed PA in indoor classrooms at the community healthcare centers, and those assigned to the NW performed the activity on outdoor trails near the healthcare centers. Each exercise program was conducted 3 times a week for 60 min per session for 6 months (Fig. 1A). The CWP includes both walking exercise and cognitive training programs and is performed indoors using a specially designed floor mat. This is a thin rubber mat that is marked with seven horizontal and five vertical lines, thus creating 24 cells (6×4 cells, 180 cm in length×80 cm in width) to step on. A number was assigned to each cell, and the participants were asked to walk forward, backward, and diagonally according to the numbers shown on the board placed in front of the room (Fig. 1B), which indicated which foot should be placed in which cell in a given sequence.
The CWP consisted of three types of walking: cardiorespiratory walking, dual-task walking, and visual memory walking. Cardiorespiratory walking involved walking patterns that were focused on improving cardiorespiratory fitness by moving the lower limbs rigorously and widely (e.g., squat, lunge, ad/abduction). Additionally, bending and straightening the knees to strengthen the lower extremity muscles and side-step motions to change the center of gravity were included. Dual-task walking involved walking patterns that required the upper and lower limbs to perform specific movements concurrently. Here, a dual task is defined as a task that requires coordination, maintenance and integration of two tasks (Wong et al., 2015). Visual memory walking involved presenting various levels and stages of walking patterns to the participants and having them perform the exercise based on memory. Three levels of stepping patterns were available for each type of walking: beginner, intermediate, and advanced, and each level consisted of 15 stages, thus providing 45 different stepping patterns. The difficulty of the 45 stepping patterns varied based on the complexity of the arm and/or leg movements required to perform within each stepping pattern. All participants in the CWP started with the easiest stepping pattern (beginner, stage 1) for each type of walking in the very first session, and they advanced to the next stage/level for each walking type once they mastered the given stepping pattern. The subjects were instructed to maintain moderate intensity based on the Borg rating of perceived exertion (RPE, scale of 12–13) by reporting their effort level every 5 min during the exercise (Eggermont et al., 2009). An instructor first demonstrated each level and stage of the stepping patterns and performed the movements along with the participants throughout the session.
The participants who were assigned to the NW-S group (i.e., controls) walked on outdoor trails together with an instructor, who was a student majoring in sports and health care at Sangmyung University. The instructor helped the participants to find directions on the trail and also to maintain moderate intensity by having them report their RPE effort level every 5 min during the walk.
Visuomotor adaptation
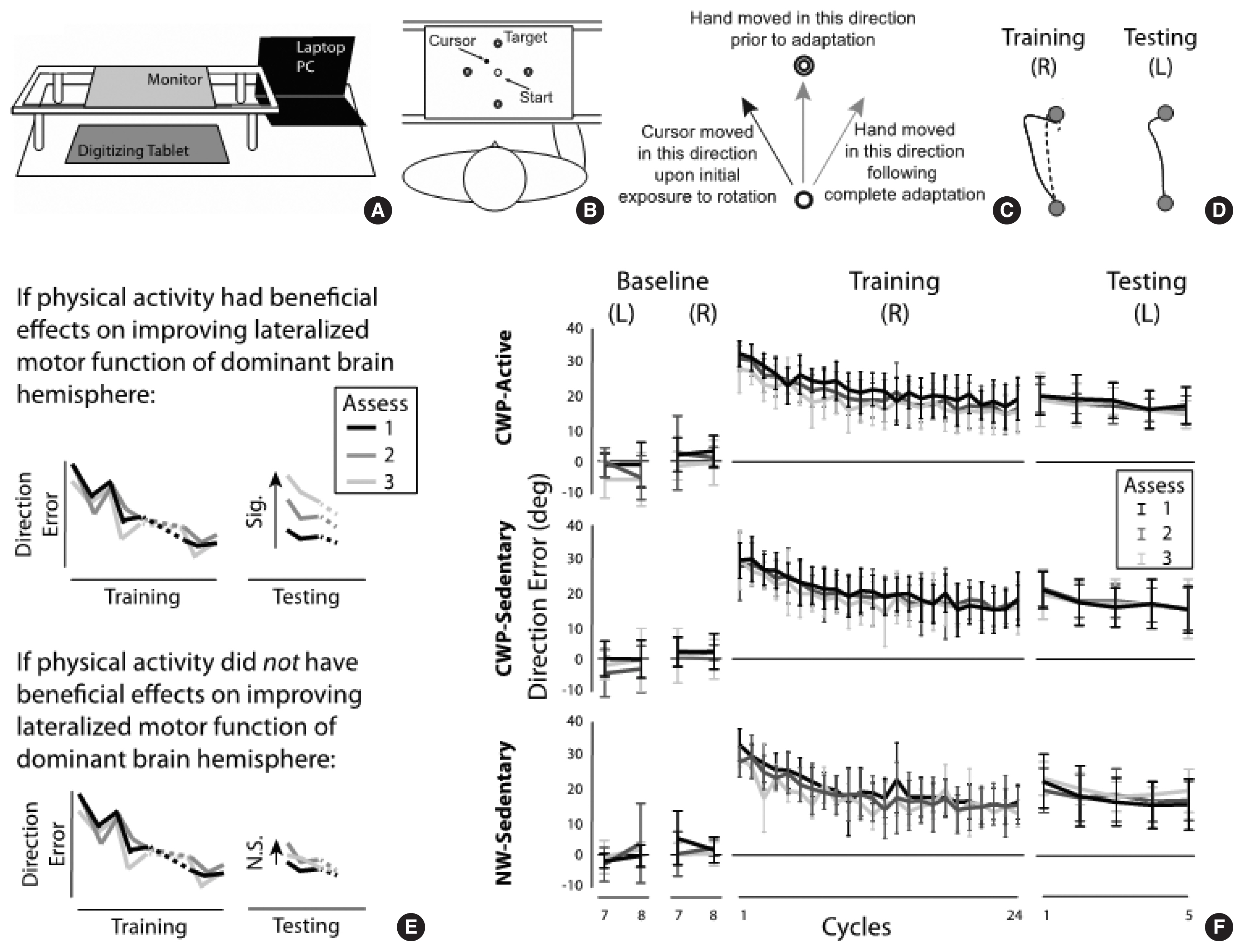
To assess motor function of the brain, the pattern of interlimb transfer following visuomotor adaptation was investigated. Fig. 2A illustrates a portable data acquisition system that comprised a laptop pc (Samsung Notebook 9 Pro, Suwon, Korea), a digitizing tablet (Wacom Intuos Pro, PTH 860, Saitama, Japan) and a monitor (GeChic 1503H, Taichung, Taiwan). The subjects performed targeted reaching movements with the index finger on the tablet. The monitor was placed above the digitizing tablet to block the direct view of the moving hand. To study adaptation to a novel visuomotor condition, a program called Presentation (NeuroBehavioral Systems, Berkeley, CA, USA) was used to rotate the visual display of a cursor representing the index fingertip location 30 degrees counterclockwise about the start circle (1 cm in diameter) during reaching movements made to one of four targets (1 cm in diameter, 10 cm from start circle) presented in a pseudorandom order (Fig. 2B).
The subjects experienced six experimental sessions: baseline with the right hand, baseline with the left hand, training with the right hand, testing with the left hand, and unlearning with the left hand. The order of the hand used in the training and testing sessions (i.e., the right arm first, then the left arm) was determined based on the previous findings (Sainburg and Wang, 2002; Wang et al., 2011; Wang et al., 2016), which indicated that healthy young adults and active older adults do not show transfer of visuomotor adaptation from the right to the left arm, but sedentary older adults do in this type of experimental setting. Transfer from the left to the right arm (i.e., training with the left arm, testing with the right arm) was not examined in this study because transfer in that direction would be expected regardless of the subject’s age and lifestyle based on the previous studies.
The baseline session (32 trials with each hand) allowed the subjects to become familiarized with the general reaching task, which required a rapid reaching movement as straight as possible from the start circle to one of the four targets. In the training session with the right arm (96 trials), the novel visuomotor rotation was provided in such a way that the hand movement made in the “12 o’clock” direction, for example, resulted in the cursor movement made in the “11 o’clock” direction (Fig. 2C). Following complete adaptation to the visuomotor perturbation, the subjects were able to bring the cursor relatively straight to the target by moving the hand in the “1 o’clock” direction (Fig. 2D). In the testing session with the left hand (20 trials), the same visuomotor perturbation was provided. In the unlearning session (48 trials with the right arm), the subjects performed reaching movements under a veridical condition (i.e., no rotation), so that they would de-adapt from the visuomotor perturbation condition. This session was provided to minimize the carryover effect of visuomotor adaptation from the 1st assessment at baseline to the 2nd assessment after 3 months to the 3rd assessment after 6 months (Fig. 1A, right panel).
Fitness tests
Cardiorespiratory fitness, lower extremity muscle strength, and active balance ability were measured to determine whether the physical fitness of the participants changed during the 6-month period. Cardiorespiratory fitness was evaluated by measuring the number of times the knee was lifted by 70 degrees for 2 min. Lower extremity muscle strength was measured by the number of times the participants sat and stood up completely from a chair for over 30 sec. Active balance ability was measured by the time required to run to a target located 3 m ahead, starting from a seated position and then returning to a seated position on the chair.
Statistical analysis
With respect to visuomotor adaptation, the performance measure of interest was direction error (DE), which was calculated as the angle between the target line (i.e., straight line from the start circle to the target) and the line defined by the start location of the hand and the point at which peak tangential velocity occurred. This was also the main performance measure in the previous studies (Sainburg and Wang, 2002; Wang et al., 2011, 2016), which demonstrated significant transfer from the nondominant left arm to the dominant right arm, but not vice versa, in terms of DE in healthy young and active older adults, but not in sedentary older adults. The comparisons of primary interest were (a) between the training and testing sessions at the first cycle in each subject group; and (b) across the three assessments at the first cycle of the testing session in each group. A significant decrease in DE from the first cycle of the training session to that of the testing session would indicate significant transfer from the right to the left arm. Significant differences in DE across the three assessments at the first cycle of the testing session would indicate that the pattern of interlimb transfer changed. It was hypothesized that if the CWP (or the NW) had beneficial effects for improving motor function of the brain in any subject group, a significant increase in DE from the first to the second and/or to the third assessment would be observed (Fig. 2E, top panel). Such results would indicate that the pattern of interlimb transfer changed from more symmetrical (i.e., transfer from right to left, as well as that from left to right (not tested in this study)) to less symmetrical or asymmetrical (i.e., lack of or less transfer from right to left), which in turn would suggest an improvement in the specialized motor function of the dominant hemisphere with participation in the given exercise program (Wang et al., 2016). No difference in DE across the three assessments at the first cycle of the testing session would indicate that the CWP or NW had no beneficial effect (Fig. 2E, bottom panel). Thus, the DE data were subjected to a repeated-measures analysis of variance (ANOVA) with group (CWP-A, CWP-S, NW-S) as a between-subject factor, and assessment (baseline, 3 months, 6 months) and cycle (the last cycle from the baseline session, the first and last cycles from the training session, the first and last cycles from the testing session) as two within-subject factors. A cycle represents the mean of four consecutive trials (i.e., movements made in all four target directions). With respect to physical fitness, a repeated-measures ANOVA was conducted with group as a between-subject factor and assessment as a within-subject factor. The alpha level was set at 0.05 for all ANOVAs and post hoc comparisons (Tukeys tests for between-group comparisons, Fisher least significant difference tests for within-group comparisons).
RESULTS
Fig. 2F depicts the changes in DEs across cycles in the baseline, training and testing sessions for the three subject groups. Gradual improvement in performance, reflected by a decrease in DEs across cycles, was observed during the training session in each of the three assessment points for all subject groups. In the testing session, DEs at the first cycle were substantially lower than those at the first cycle of the training session, again in each of the three assessment points for all subject groups. The repeated-measures ANOVA indicated a significant main effect of cycle (P<0.001). The main effect of group or assessment was not significant (P=0.824 and P= 0.138, respectively); and no interaction effect was significant (P> 0.05 for all interaction effects). Post hoc comparisons were done between the first cycle of the training session and that of the testing session, which indicated a significant difference (P=0.003), that is, a significant transfer of visuomotor adaptation from the right to the left arm.
With respect to physical fitness, the ANOVA indicated a significant group×assessment interaction for cardiorespiratory fitness and active balance ability (P=0.003 and P=0.004, respectively). Post hoc analyses indicated a significant improvement between the 1st and 2nd assessments, and between the first and 3rd assessments for all three groups, but at different rates, in terms of both cardiorespiratory fitness and active balance ability (P<0.05). For lower extremity muscle strength, the main effect of time was significant (P<0.001), indicating an improvement over time. Neither the main effect of group nor the group × assessment interaction was significant for lower extremity muscle strength (P=0.79 and 0.817, respectively). Collectively, these results indicate a general improvement in physical fitness in all subject groups over the 6-month period.
DISCUSSION
Visuomotor adaptation is a type of motor learning that is studied extensively in the field of systems neuroscience (Krakauer et al., 2000; Pine et al., 1996; Wang and Sainburg, 2005). When individuals are exposed to a visuomotor perturbation (e.g., a rotated visual display) for the first time, their hand-paths become largely curved. As they perform reaching movements repeatedly under the same visuomotor condition, their hand-paths become relatively straight to the targets, indicating adaptation to the given condition. Such adaptation is thought to be associated with the formation of an internal representation of the given visuomotor condition in the cerebellum (Bernard and Seidler, 2013; Ghilardi et al., 2000).
Interlimb transfer of visuomotor adaptation has been studied extensively. Sainburg and Wang (2002) and Wang and Sainburg (2006) demonstrated that transfer of visuomotor adaptation, in terms of directional control, occurred from the nondominant to the dominant arm, but not from the dominant to the nondominant arm in both right and left handers. The authors concluded that the dominant hemisphere/limb system is specialized for controlling directional features of reaching movements, and also that transfer occurs asymmetrically because of the lateralized motor function of each brain hemisphere. Such asymmetrical transfer of visuomotor adaptation has been observed in physically active older adults, but not in sedentary older adults, indicating a decline in the lateralized motor function of the brain in sedentary, but not active, older adults (Wang et al., 2016). Based on these findings, we hypothesized in the current study that if the CWP had beneficial effects for improving motor function of the brain, the extent of transfer from the right to the left arm would decrease from the first to the second and/or to the third assessment (e.g., significant transfer at the first assessment, nonsignificant transfer at the third assessment), especially in the sedentary group. Our results showed that significant transfer of visuomotor adaptation occurred from the right to the left arm in all subject groups at the first assessment; and the participants’ physical fitness improved with the progress of the CWP as well. Despite that, the DEs at the first cycle of the testing session did not change across the three assessment points in any subject group, indicating that the same extent of interlimb transfer was maintained throughout the 6-month long participation in the CWP (and also the NW). These results suggest that participating in the CWP (or the NW) for 6 months did not have any positive influence on the motor function of the dominant hemisphere in the subjects tested in this study.
It should be noted that transfer from the right to the left arm was observed not only in sedentary, but also in active older adults in the current study, which was not the case in the study reported by Wang et al. (2016). This discrepancy between the two sets of findings may be due to the fact that the ‘active’ older adults tested in the current study may not have been so active. In the current study, the participant’s lifestyle was determined using the SBAS, which has demonstrated validity for assessing frequency and intensity of leisure time PA (Taylor-Pillae et al., 2010). The SBAS was used in the study of Wang et al. (2016) as well. In that study, however, the authors also measured the PA level of the subjects by having them wear an accelerometer for 7 days, ensuring that their self-report was correct. This was not done in the current study, which is the limitation of this study; and thus, it is possible that the self-report of the subjects tested in this study was not entirely correct. This argument is supported by the physical fitness results in the current study, which showed no significant difference between the active and sedentary subjects in terms of their cardiorespiratory fitness, lower extremity muscle strength and active balance ability at the baseline assessment (P>0.05). These results indicate that the participants who were classified as active and those classified as sedentary had a similar level of physical fitness prior to their participation in the CWP, which possibly resulted in the similar pattern of interlimb transfer. Nonetheless, the aforementioned limitation does not seem to have influenced the main finding of this study in any significant way, because our physical fitness data indicate that all our subjects were basically ‘sedentary’; and our hypothesis was most appropriate for sedentary older adults.
Though not beneficial for improving motor function of the brain, the CWP has been shown to improve cognitive function in healthy older adults. Kang et al. (2021) investigated the effects of the CWP on cognitive function in both active and sedentary older adults using the same exercise protocol (60 min per session, 3 times a week, for 6 months; cognitive function measured at baseline, 3 months, and 6 months after program onset). Cognitive function was evaluated using the Seoul Neuropsychological Screening Battery (SNSB-II) (Lee et al., 2018) by certified clinical psychologists who had at least 3 years of experience with administering the SNSB-II. Scores for attention, visuospatial function, memory, and frontal/executive function were obtained. The results revealed significant improvements in visuospatial function, memory, and frontal/executive function over time, and the extent of improvement from the baseline to the 6-month assessment point was greater in the active, as compared to the sedentary, participants for attention, memory and frontal/executive function. These findings indicate the beneficial effects of the CWP for improving cognitive function in older adults, especially those who maintain active lifestyle. These findings related to the positive effects of the CWP on cognitive function are consistent with the findings reported by Teixeira et al. (2013), who examined the beneficial effects of an exercise program, called square stepping exercise. In fact, the CWP was developed based on the idea of square stepping exercise (Shigematsu et al., 2008), which involves various walking movements in multiple directions on a thin mat. Square stepping exercise has been shown to be moderately effective for improving strength and balance, as well as cognitive function in older adults (Shigematsu et al., 2008; Teixeira et al., 2013). The CWP is a variation of square stepping exercise that has been modified to maximize its beneficial effects for cognitive function improvement by including dual-task movements, which require the upper and lower limbs to perform different types of movements concurrently. Dual-task paradigms, known to impose additional cognitive demands in the brain, have also been shown to increase brain activity in older adults (Wong et al., 2015).
Taken together, the CWP is beneficial for improving cognitive function in older adults, regardless of whether they are physically active or sedentary. This exercise program, however, does not seem to have an effect strong enough to influence motor function of the brain, at least when the motor function is reflected as changes in the pattern of interlimb transfer following visuomotor adaptation or when the duration of the program is only 6 months. The current findings suggest that one’s lifestyle (i.e., physically active or sedentary) may contribute to a reduction in hemispheric asymmetry observed in older adults (Cabeza et al., 2004; Dolcos et al., 2002; Rypma et al., 2001; Wang et al., 2016), and that a positive change in the reduced motor function of the hemispheres may require a change in their lifestyle that is maintained for a relatively long period of time (e.g., much longer than 6 months).