INTRODUCTION
Damage to the sciatic nerve, which transmits all signals form the lumbar spinal cord to the lower extremity, is mainly caused by lumbar disc herniation, compression of piriform and traffic accident (Kumar et al., 2011). And it has been well known that patients with sciatic nerve injury (SNI) suffer from poor quality life due to paresthesia loss of muscle power, and chronic peripheral neuropathic pain (Schaefer et al., 2014). Although a variety of SNI-induced symptoms are spontaneously improved in a few months post injury, the complete recovery of motor and sensory functions is quite difficult and slow (Battiston et al., 2009; Burnett and Zager, 2004). In particular, recovery of chronic neuropathic pain by nerve injury requires more time and complex therapeutic approaches compared to axonal regeneration (Sandy-Hindmarch et al., 2022).
Looking at previous studies on neuropathic pain, Wnt/β-catenin signaling pathway, tumor necrosis factor alpha (TNF-α), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) are related with neuropathic pain and highly expressed in the dorsal root ganglion cells (DRG) and spinal cord after denervation of the peripheral nerves (Itokazu et al., 2014; Leung and Cahill, 2010). And pharmacological inhibition of pain-related molecules can result in decrease of neuropathic pain induced by peripheral nerve injury (Friedman, 2000; Quintans et al., 2014; Valsecchi et al., 2008). But these therapeutic approaches have a large economic burden and side effect compared to their effectiveness.
To solve these problems, many researchers who have studied neuropathic pain treatment emphasize the importance of regular physical activities including walking, running, jogging and swimming exercise, which is an economical therapeutic method with the fewest side effects for pain treatment (Kami et al., 2017; Mannerkorpi and Henriksson, 2007). Cobianchi et al. (2013) reported that treadmill exercise application after SNI increased axonal growth in the distal segment of the injured sciatic nerve through activation of brain-derived neurotrophic factor (BDNF), extracellular signal-regulated kinase 1/2, glial-derived neurotrophic factor, and neurotrophin-3 proteins (Seo et al., 2009). López-Álvarez et al. (2015) also represented that exercise-increased calcitonin gene-related peptide reduced mechanical allodynia after spinal cord and peripheral nerve injury. But, according to a study published by Sheahan et al. (2015), it suggested that voluntary wheel running did not attenuate functional recovery and neuropathic pain after SNI. Thus, we believe that the effect of exercise on sciatic nerve regeneration and pain relief is still controversial.
Considering these previous findings, exercise is a closely related to neuropathic pain and nerve regeneration after nervous system injury. However, research on neuropathic pain and nerve regeneration-related signals according to the timing point of exercise intervention after SNI are still insufficient. Therefore, the purpose of this study focuses on investigating whether the time point of exercise application regulates neuropathic pain and nerve regeneration-related signaling pathway in ipsilateral lumbar 4 (L4) to 6 (L6) DRG after SNI.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (5 weeks old) were used in this experiment. They were randomly divided into five groups: the normal control (CON, n=10) group, SNI+sedentary (IS, n=10), exercise+SNI (EI, n=10), SNI+exercise (IE, n=10), and exercise+ SNI+exercise (EIE, n=10) groups. Animals were maintained at a constant room temperature of 22°C–24°C and 60% of humidity under 12/12-hr light-dark cycle. They were accepted to eat commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. This experiment obtained approval by the Ethics Committee of Jeju National University (2021-0018).
Sciatic nerve injury
Rats in the injury groups were anesthetized with using an animal inhalation narcosis control (Jeungdo Bio & Plant, Seoul, Korea). The rats were placed into the chamber with 2%–2.5% concentration of isoflurane for anesthesia and then 1.5%–1.8% concentration for maintenance during SNI. The left sciatic nerve was crushed with a pair of forceps held tightly for 1 min and 30 sec at intervals (Cho et al., 2021). After surgery, anesthetized animals were then placed on a heating pad maintained at 37°C, and then they were put in their cages for resting. Rats in IE group received a crush injury of the sciatic nerve before of treadmill exercise, and EI and EIE groups were performed SNI after training.
Treadmill exercise protocols
To adapt the treadmill walking, all rats in this study performed low intensity treadmill exercise for a week before the study began. Treadmill device (Jeungdo Bio & Plant) was used for rats only, and the animals in the exercise groups were subjected to walking at a speed of 8 m/min for 30 min with no inclination. EI and IE groups walked on the treadmill before or after SNI for 14 days, respectively. And rats in EIE group performed treadmill exercise both before and after SNI for 14 days. IS group were rested in the cage for 14 days after SNI. All rats were sacrificed 2 days after the end of the experiment.
Western blot analysis
The dissected L4 and L6 DRG were rinsed with phosphate-buffered saline (PBS) and lysed in Triton lysis buffer. Being denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4°C. Protein (20 μg) was used for Western blot analysis using anti-Wnt3a rabbit polyclonal antibody (1:1,000, GeneTex Inc., Irvine, CA, USA), anti-β-catenin mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-growth associated protein 43 (GAP-43) mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-NF-κB mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-TNF-alpha rabbit polyclonal antibody (1:1,000, Sino Biological, Wayne, PA, USA), anti-BDNF mouse monoclonal antibodies (1:1,000, Santa Cruz Biotechnology). For the secondary antibody, horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (1:1,000, GeneTex Inc.) were used. The blotting proteins were detected by using Westar ECL substrates (Cyanagen, Bologna, Italy). Detected band intensity was analyzed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Immunofluorescence staining
L4 and L5 DRG were embedded and frozen at 20°C. Transverse (10 μm thick) were cut on a cryostat and mounted on positively charged slides (Fisher Scientific, Pittsburgh, PA, USA). For double immunofluorescence staining sections were fixed with 4% paraformaldehyde and 4% sucrose in PBS at room temperature for 40 min, permeabilized with 0.5% Nonidet P-40 in PBS, and blocked with 2.5% horse serum and 2.5% bovine serum albumin for 4 hr at room temperature. The sections were incubated with anti-GAP-43 mouse polyclonal antibody (1:200, Santa Cruz Biotechnology), anti-Neurofilametn-200 mouse monoclonal antibody (1:200, Sigma-Aldrich, Taufkirchen, Germany) anti-Wnt3a rabbit polyclonal antibody (1:400, GeneTex Inc.), anti- anti- β-catenin mouse monoclonal antibody (1:400, Santa Cruz Biotechnology), anti-NF-κB mouse monoclonal antibody (1:200, Santa Cruz Biotechnology), anti-TNF-alpha rabbit polyclonal antibody (1:500, Sino Biological). Then they were incubated with rhodamine-goat anti-rabbit secondary antibody (1:400, Molecular Probes, Eugene, OR, USA) or fluorescein-goat anti-mouse antibody (1:400, Molecular Probes) and Hoechst (1:1,500, Sigma-Aldrich) for 1 hr at room temperature, and cover-slipped with gelatin mount medium. The stained samples were viewed with a fluorescence microscope (Nikon model E-600, Nikon, Kawasaki, Japan), and the images were captured with a digital camera, and analyzed using Adobe Photoshop Software (version CS6, San Jose, CA, USA).
RESULTS
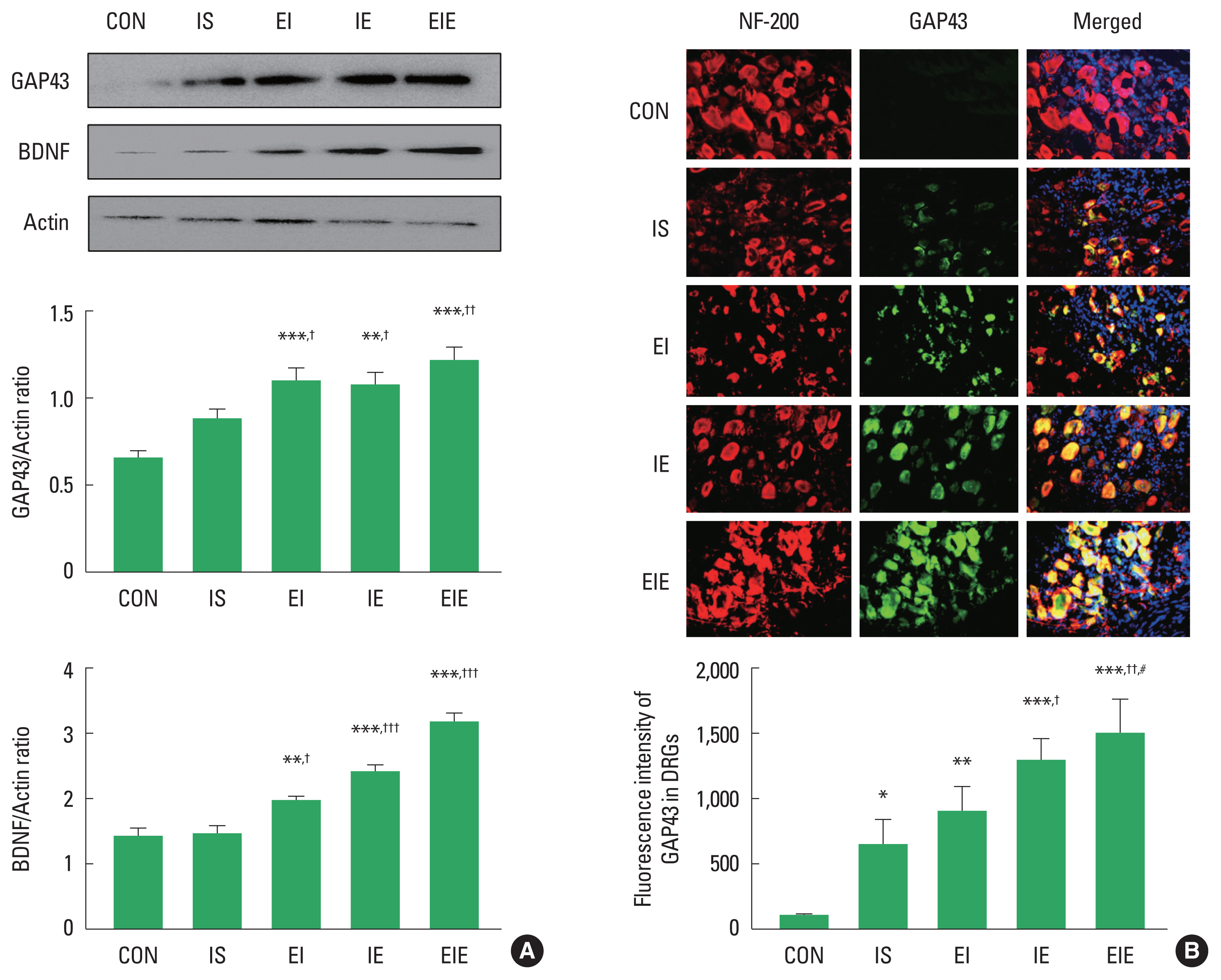
The timing point of exercise application regulated expression levels of GAP-43 and BDNF
GAP-43 and BDNF have been known as a specific axon regeneration maker in the injured peripheral nervous system and they are anterogradely transported from DRG toward injured axon. To determine the difference in axon regeneration-related proteins according to the timing point of treadmill exercise intervention after SNI, we analyzed induction levels of GAP-43 and BDNF in L4–L6 DRG of the ipsilateral side. As shown in Fig. 1A, GAP-43 and BDNF were quantitatively and significantly increased in the EI, IE and EIE groups compared to the IS group, suggesting that exercise application before and/or after SNI may be positive modulator for sciatic nerve regeneration. For a histological examination in DRG, the ipsilateral L4–L6 DRG were stained with neurofilament-200 (NF-200), GAP-43 and Hoechst. NF-200 antibody is a marker of primary sensory neuron and Hoechst is a popular cell-permeant nuclear counterstain. Colocalization of GAP-43 with NF-200 was not only higher in the IE and EIE groups than those seen in the IS group, but also EIE group showed higher fluorescence intensity of GAP-43 than EI group (Fig. 1B), suggesting that exercise application after SNI may increase GAP-43 immunoreactivity in the ipsilateral DRG.
Expression of Wnt3a and β-catenin at the timing point of exercise application
Wnt/β-catenin signaling have been known as activated in dorsal horn and DRG after sciatic nerve ligation (Zhao and Yang, 2018). To examine the timing point of training on neuropathic pain-related proteins in ipsilateral DRG at the L4–5 after SNI, we analyzed expression levels of Wnt3a and β-catenin in the DRG whole cell using Western blot and immunofluorescence staining techniques. As shown in Fig. 2A, Wnt3a and β-catenin proteins were quantitatively and significantly decreased in the IE and EIE groups compared to the IS group. To obtain the qualitative results of neuropathic pain-related proteins in the ipsilateral L4–6 DRG, we applied immunohistochemistry experiment. Colocalization of Wnt3a with β-catenin was lower in the IE and EIE groups than those seen in the IS group (Fig. 2B), suggesting that exercise application after SNI may decrease Wnt3a and β-catenin immunoreactivity in the ipsilateral DRG.
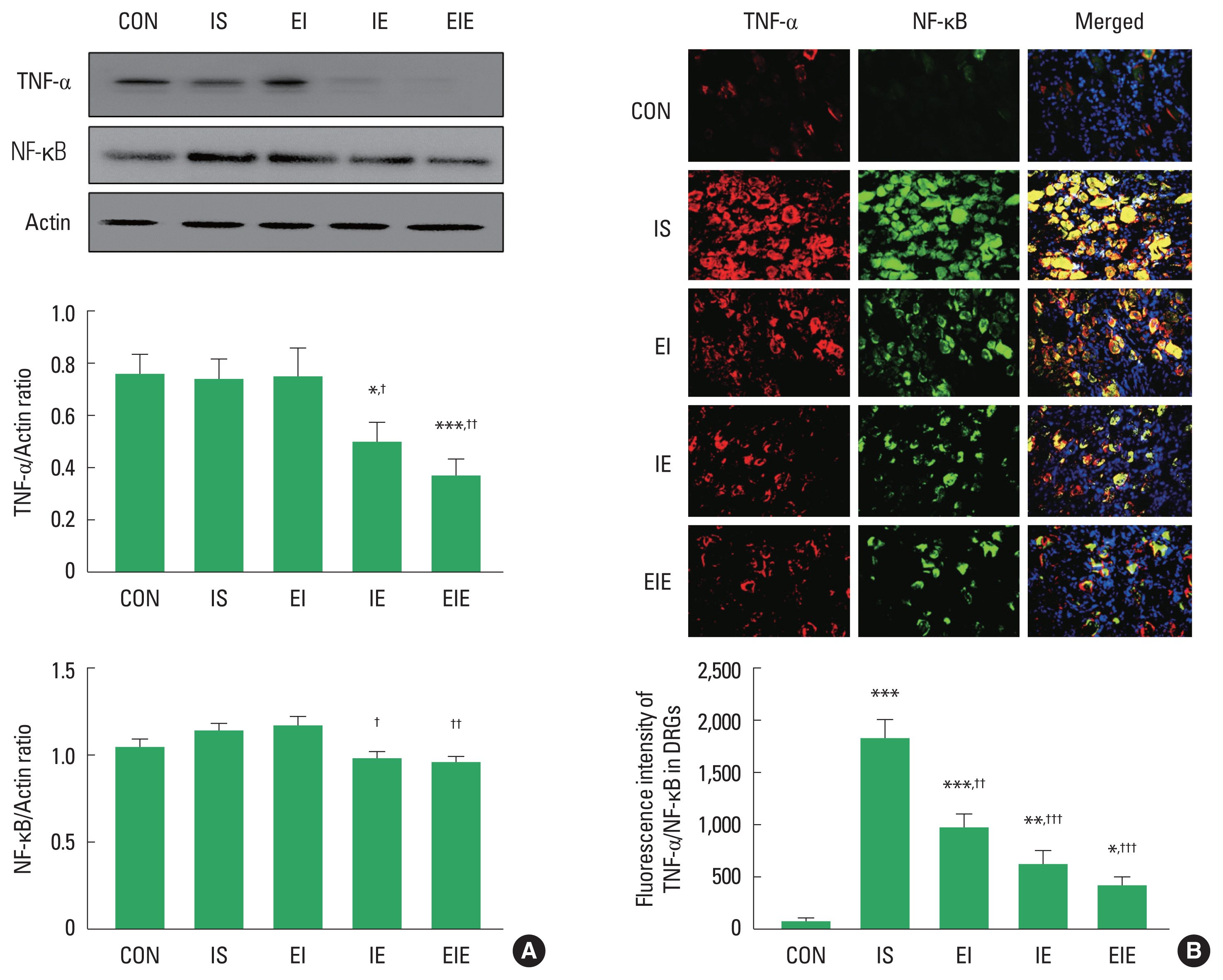
Expression of NF-κB and TNF-α at the timing point of exercise application
NF-κB and TNF-α have been known as participating in regulating the inflammation and pain of the injured peripheral nerve. To confirm changes in NF-κB and TNF-α expression after SNI, we analyzed NF-κB and TNF-α at the ipsilateral L4–6 DRG. As shown in Fig. 3A, IE and EIE groups were significantly downregulated in NF-κB expression level compared to the IS group. TNF-α was also significantly decreased in IE and EIE groups than the IS group. In the immunohistochemistry images, colocalization of NF-κB and TNF-α were lower in the EI, IE and EIE groups than those seen in the IS group (Fig. 3B).
DISCUSSION
The injured peripheral nerves undergo Wallerian degeneration, which is separation and myelin clearance of distal ends of the nerve (Gaudet et al., 2011). The sciatic nerve belonging to the peripheral nervous system can spontaneously regenerate after injury, but recovery rate is quite low. GAP-43 and BDNF are dramatically upregulated in the injured sciatic nerve axons and DRG, which has been well known as a crucial marker for peripheral nerve regeneration (Carriel et al., 2017; Lopes et al., 2017). McGregor and English (2019) suggested that BDNF should promote the survival of motor neurons in the ventral horn of the spinal cord, and sensory neuron regeneration in DRG as well as axonal regrowth after peripheral nerve injury. Also, GAP-43 has been known to be anterogradely transported from DRG neurons to improve motor and sensory functions after peripheral nerve injury (Buzoianu-Anguiano et al., 2020; Donnelly et al., 2013). In the present study, we confirmed that exercise before and/or after SNI significantly GAP-43 induction level in DRG of the ipsilateral side compared to sedentary groups. Previous studies reporting on nerve regeneration and regular exercise suggested that that neurotrophin 4/5 and BDNF were required for the early stage of regeneration axons in peripheral nerves, and they were continuously increased for a 4weeks treadmill exercise after SNI (English et al., 2005). In addition, Kim et al. (2020) investigated that low-intensity treadmill exercise upregulated expression level of GAP-43 in the injured axons and ipsilateral DRG after SNI. These findings in previous studies support our findings that regular treadmill walking might be a valid therapeutic method for facilitating axonal regrowth in the injured sciatic nerve. But, we believe that additional studies are necessary to understanding the specific biochemical mechanism of various regenerative signaling pathways at the time point of exercise application.
Recent studies on neuropathic pain have confirmed increase of β-catenin and Wnt3a in DRG after L5 spinal nerve ligation and chronic constriction injury (Zhang et al., 2013), suggesting the importance of blocking the Wnt/β-catenin signaling pathway to minimize neuropathic pain after nerve injury (Itokazu et al., 2014). However, the research findings on relationship between the timing point of exercise intervention and Wnt/β-catenin signaling pathway in the ipsilateral L4–L6 DRG after SNI is still insufficient. Thus, we investigated activation of Wnt/β-catenin signaling pathway in the ipsilateral DRG, and confirmed that IE and EIE groups sharply downregulated Wnt3a and β-catenin levels than CON and IS groups, but EI group did not show a significant difference when compared to the other groups. Grace et al. (2016) and Tsai et al. (2017) emphasized that preoperative or postoperative exercise might have a positive effect on regulating the expression level of interleukin-6, TNF-α and Wnt/β-catenin in injured sciatic nerve and downregulation of this signaling pathway could relieve neuropathic pain induced by SNI (Cho et al., 2021). Our results regarding reduction of the Wnt/β-catenin signaling activity by exercise before and/or after SNI were partially consistent with previous studies suggesting that Wnt/β-catenin pathway can modulate neuropathic pain.
TNF-α, an indicator for neuropathic pain, is known to contribute to pain sensitivity and death of neuronal and nonneuronal cells in the injured central and peripheral nervous system (Baud and Karin, 2001; Schäfers et al., 2003). Low-intensity physical activities including swimming and walking decreased TNF-α induction in the injured axons and ipsilateral DRG after SNI for regulating neuropathic pain (Chen et al., 2012). In the present study, expression of NF-κB and TNF-α proteins in the ipsilateral L4 to L6 DRG were dramatically decreased in IE and EIE groups when compared to other groups, implying that exercise application after SNI might be more effective for reducing pain sensitivity in DRG than exercise application before SNI. According to some previous studies, both pre- and postoperative exercises significantly decreased TNF-α, interleukin-β and 6R levels in the axons and DRG after peripheral nerve injury (Cox et al., 2017; Kakihata et al., 2016). Additionally, aerobic exercise was decreased mechanical hypersensitivity and proinflammatory cytokine levels regardless of the time of exercise application (Bobinski et al., 2011).
Given these results obtained in previous and present studies, the timing point of treadmill exercise application would positively regulate neuropathic pain in the ipsilateral L4–6 DRG after SNI. In conclusion, our findings suggest new evidence that exercise before and/or after SNI might be a therapeutic method for facilitating axonal regeneration and relieving neuropathic pain.