INTRODUCTION
Sciatic nerve injury (SNI) has been known to be caused by traumatic pressure, stretching and cutting to the nerve during sports, and to result in serious clinical problems including motor and sensory deficits as well as neuropathic pain (Cho and Seo, 2022). Therefore, many previous researches on nerve regeneration have focused on behavioral improvement through facilitating axonal regrowth and neuropathic pain relief, and they have tried to reduce the regeneration period required for injured axons to reach the target skeletal muscles (Höke and Brushart, 2010).
Among the various therapeutic methods that can promote peripheral nerve regeneration, regular walking, swimming, cell transplantation, and drug treatment are main approaches to induce positive histological and functional changes in Schwann cells (Klimaschewski et al., 2013; Sarikcioglu and Oguz, 2001; Zainul et al., 2022). In particular, transplantation of autologus bone marrow stromal cell (BMSC) increases induction levels of nerve fiber regrowth and pain-related proteins in the injured nerve and dorsal root ganglion (DRG) after SNI (Guo et al., 2011; Matsuda et al., 2017), and these regenerative response is influenced not only by activating neurotrophic factors including neurotrophin-3, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and ciliary neurotrophic factor (CNTF) (Wang et al., 2014) but also by regulating specific proinflammatory cytokines including tumor necrosis factor (TNF)-α, nuclear factor (NF)-κB, interleukin (IL)-6) and tropomyosin-receptor kinase B (TrkB) receptor (Yang et al., 2018; Zhai et al., 2020). Also, Teng et al. (2019) provided reliable evidence that BMSC treatment could improve mechanical hyperalgesia and allodynia in long-term denervated rat without ethical problems and side effects (Guo et al., 2018; Huang et al., 2017).
It has been reported that sustaining physical exercise such as walking and swimming might accelerate functional recovery of rat hindlimbs with SNI (Cho and Seo, 2022) and downregulate neuropathic pain-related protein expression in the ipsilateral DRG (Martins et al., 2018). Furthermore, Almeida et al. (2015) demonstrated that regular exercise could improve neuropathic pain symptoms such as thermal and tactile hyperalgesia and allodynia through activation of BDNF after sciatic nerve ligation. For these reasons, many researchers in the field of exercise physiology and neuroscience have suggested that regular physical exercise might be one of therapeutic strategies for facilitating elongation of sciatic nerve axons and alleviating neuropathic pain after SNI.
Considering these previous findings on regeneration, a single effect of physical exercise or BMSC transplantation on axonal growth and pain relief was identified. However, therapeutic mechanism of treadmill exercise combined with BMSC transplantation after SNI is not clear. In addition, there are not enough studies reporting long-term effects from the early to late stages of nerve regeneration. Therefore, the purpose of present study was to investigate whether combined intervention of physical exercise and BMSC transplantation would affected expression of neurotrophic factors in the injured sciatic nerve and neuropathic pain-related cascades in ipsilateral DRG during the early or late stage of sciatic nerve regeneration.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (6 weeks old, n=102) were randomly divided into the normal control group (CONT, n=6), sedentary group (SS, n=24), exercise group (SE, n=24), BMSC transplantation group (SB, n=24), BMSC transplantation+exercise group (SBE, n=24) 1, 2, 3, and 5 weeks after SNI. Animals were maintained at a constant room temperature of 22ºC–24ºC and 60% of humidity under 12/12-hr light-dark cycle. They were accepted to eat commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. This experiment obtained approval by the Ethics Committee of Jeju National University (2019-0028).
Sciatic nerve injury
Rats in the injury groups were anesthetized with using an animal inhalation narcosis control (Jeungdo Bio & Plant, Seoul, Korea). Following the surgical procedure reported in Cho’s study (Cho and Seo, 2021), all rats were placed into the chamber with 2%–2.5% concentration of isoflurane for anesthesia and then 1.5%–1.8% concentration for maintenance during SNI. The left sciatic nerve was crushed with a pair of forceps held tightly for 1 min and 30 sec at intervals (Seo et al., 2021). After surgery, anesthetized animals on a heating pad were put in their cages for resting. Operated rats were allowed to rest for 2 days in cages.
Treadmill exercise protocols
All animals took part in this experiment had an adaptation period to treadmill exercise for 1 week. Treadmill exercise (Jeungdo Bio & Plant) was applied at 8 m/min for 30 min with no inclination for each exercise intervention period (week 1, 2, 3, and 5 after postinjury). All exercise groups were sacrificed 2 days after the end of the experiment.
BMSC culture and transplantation
As suggested in Cho’s study (Cho and Seo, 2021), the femur and tibia of 4-week-old SD rats were dissected, and bone marrow tissue was extracted using Dulbecco’s modified Eagle’s medium (DMEM) medium. Extracted bone marrow tissue were cultured in DMEM medium with 20% fetal calf serum. One week after cell culture, BMSCs adhered to the base on the culture dishes proliferated to a density of ca. 5×106 in one dish. Single dose of 5×106 harvested BMSCs (30-μL phosphate-buffered saline) was injected into the injury area using a 30-gauge needle.
Western blot analysis
The dissected lumbar 4 and 5 (L4 and L5) DRG and sciatic nerve tissue were rinsed with phosphate-buffered saline and lysed in Triton lysis buffer. Being denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4ºC. Protein (20 μg) was used for Western blot analysis using anti-TrkB rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology, Danvers, MA, USA), anti-β-actin mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-NF-κB mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-CNTF mouse monoclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-NGF mouse monoclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-BDNF mouse monoclonal antibodies (1:1,000, Santa Cruz Biotechnology), anti-TNF-α rabbit polyclonal antibody (1:1,000, Sino Biological, Wayne, PA, USA), anti-IL-6 rabbit polyclonal antibody (1:1,000, GeneTex Inc., Irvine, CA, USA). For the secondary antibody, Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (1:1,000, GeneTex Inc.) were used. The blotting proteins were detected by using Westar ECL substrates (Cyanagen, Bologna, Italy) and detected band intensity was analyzed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All the data is presented as a mean±standard error. The normality of distribution based on the Shapiro–Wilk test and the parametric tests for the investigated variables were used to analyze the data. Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test. The significance level was set at P<0.05. All data analysis and graphs were by using Prism 6 (GraphPad, La Jolla, CA, USA).
RESULTS
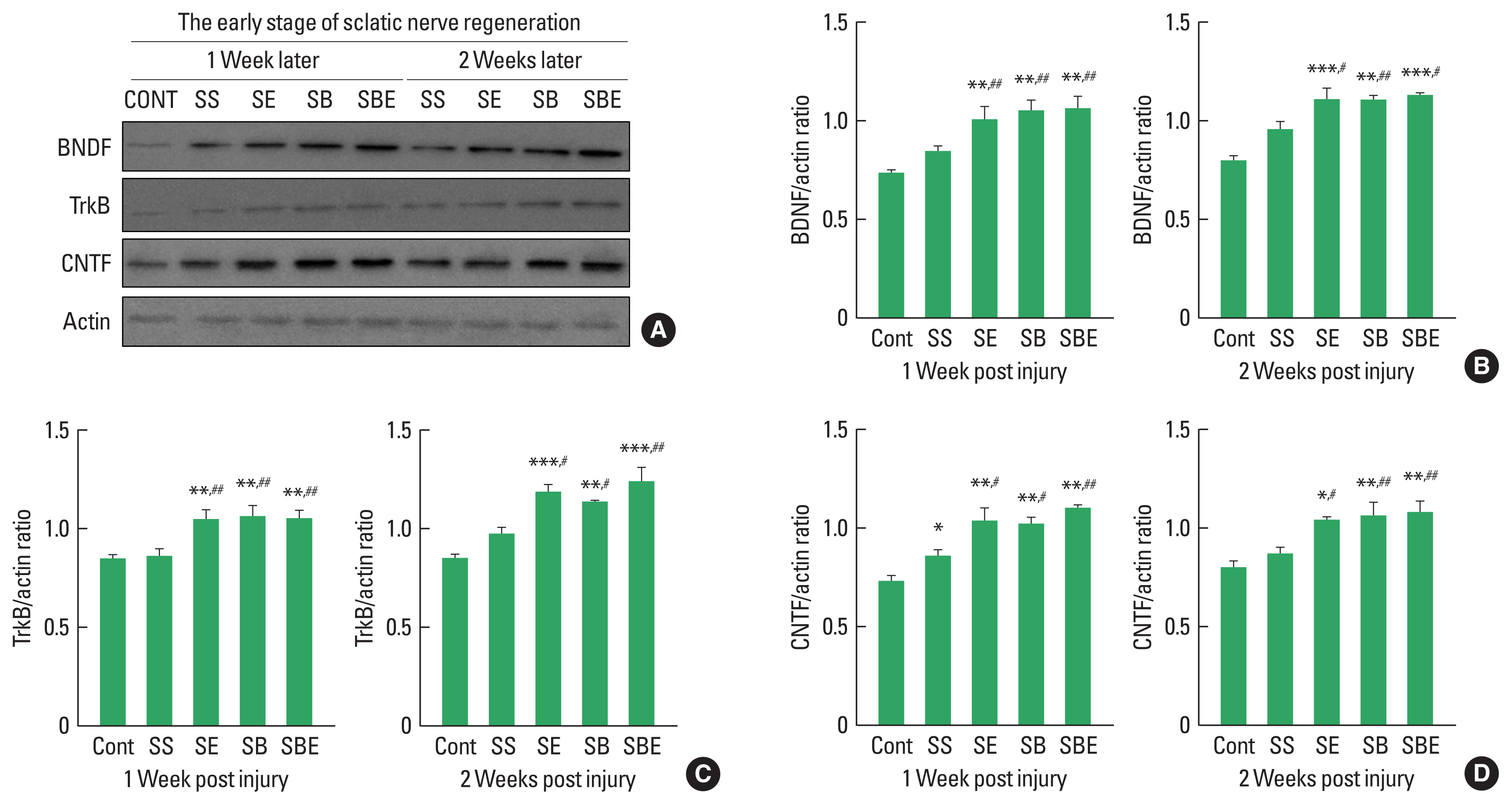
Neurotrophic factors at early stages of sciatic nerve regeneration
BDNF-TrkB signaling and CNTF are specific neurotrophic factors that are upregulated in regenerating sciatic nerve and these expression level is stimulated by aerobic exercise and BMSC transplantation after SNI. To determine effect of combined application of treadmill exercise and BMSC transplantation on BDNF-TrkB and CNTF induction levels in the injured sciatic nerve at early stage of axonal regeneration, we applied quantitative analysis using Western blotting technique. As shown in Fig. 1, at early stage of nerve regeneration, TrkB, BDNF, and CNTF were significantly upregulated in the SE and SBE groups at week 1 and week 2 postinjury than those in the CONT and SS groups, but there was no meaningful difference of TrkB, BDNF, and CNTF expression among SE, SB, and SBE groups at week 1 and 2 later (Fig. 1B–D).
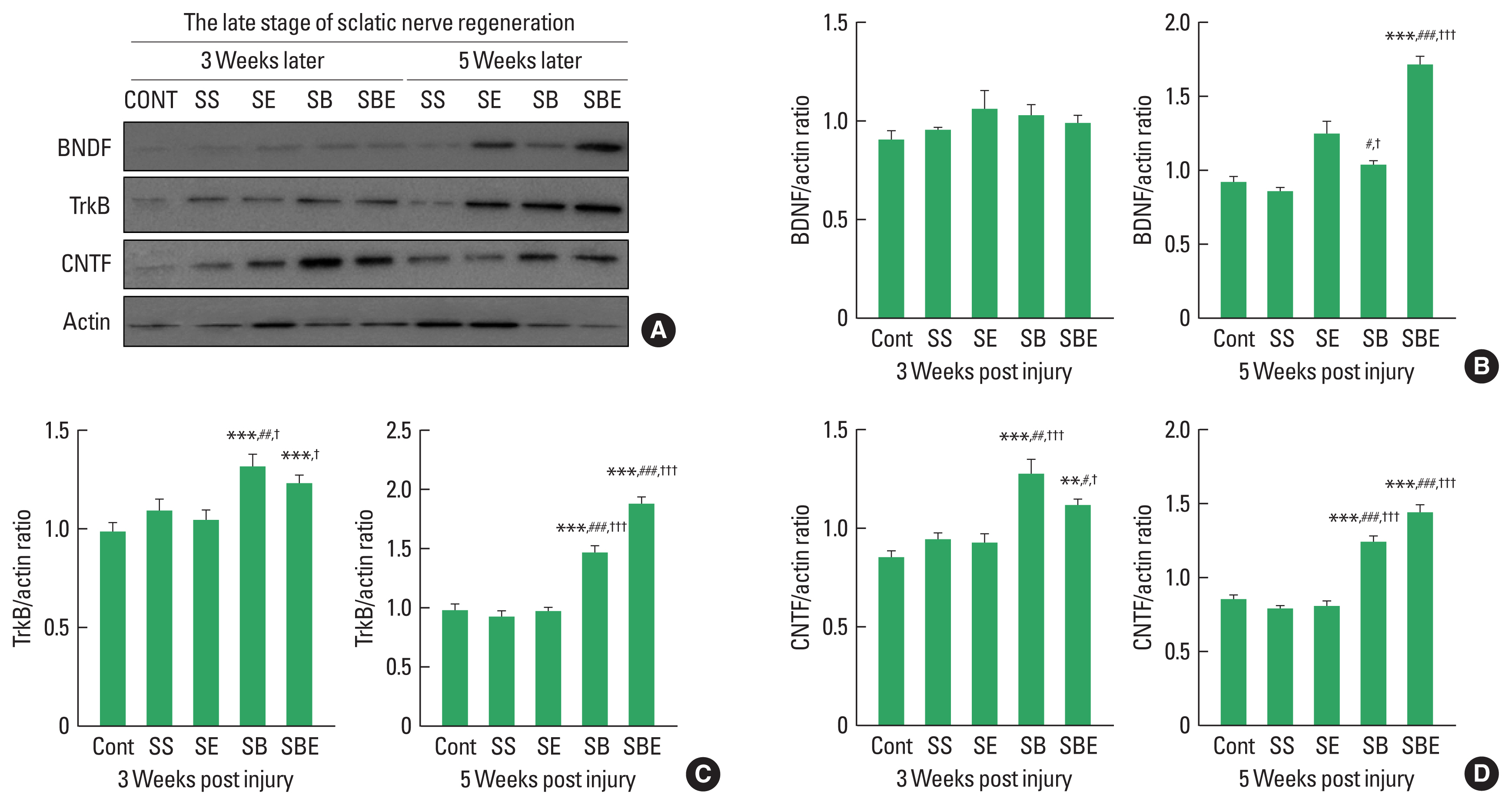
Neurotrophic factors at late stages of sciatic nerve regeneration
In the late period of peripheral nerve regeneration, remyelination is mainly induced via differentiation of Schwann cells in the distal portion of the injury site, and the process of this remyelination occurs about week 3 after SNI (Raducan et al., 2013; Seo et al., 2009). As shown in Fig. 2, at late stage of regeneration, BDNF was not significant difference in all groups at week 2 later, but SBE showed a dramatic upregulation at week 5 alter than the CONT, SS, SE, and SB groups (Fig. 2A, B). In addition, TrkB and CNTF were further enhanced in the SB and SBE groups at week 3 and week 5 later than those in the CONT, SS, and SE groups (Fig. 2C, D), whereas SE group did not significantly increased levels of TrkB and CNTF at 2 timepoints (Fig. 2C, D), suggesting synergistic effect of combined approaches on facilitating regeneration-related neurotrophic factors.
Pain-related molecules in DRG at early stages of regeneration
Proinflammatory cytokines including NF-κB, IL-6, and TNF-α are related to neuropathic pain and low-threshold mechanical allodynia, and these cytokines has been known to be rapidly accelerated in ipsilateral DRG after peripheral nerve injury. To confirm effect of combined application on changes in neuropathic pain-related cytokines in L4–5 DRG at early stage of sciatic nerve regeneration, Quantitative analysis on NF-κB, IL-6, and TNF-α was performed at week 1 and week 2 after SNI. As shown in Fig. 3, NF-κB, TNF-α, and IL-6 expression levels were considerably upregulated in all groups at week 1 and week 2 later than CONT group, and SE and SBE groups significantly downregulated induction of NF-κB, TNF-α, and IL-6 in ipsilateral L4–5 DRG at early period of axonal regeneration (Fig. 3B–D). In specific, SBE group led to downregulation in NF-κB compared to the SE group at week 2 postinjury (Fig. 3C).
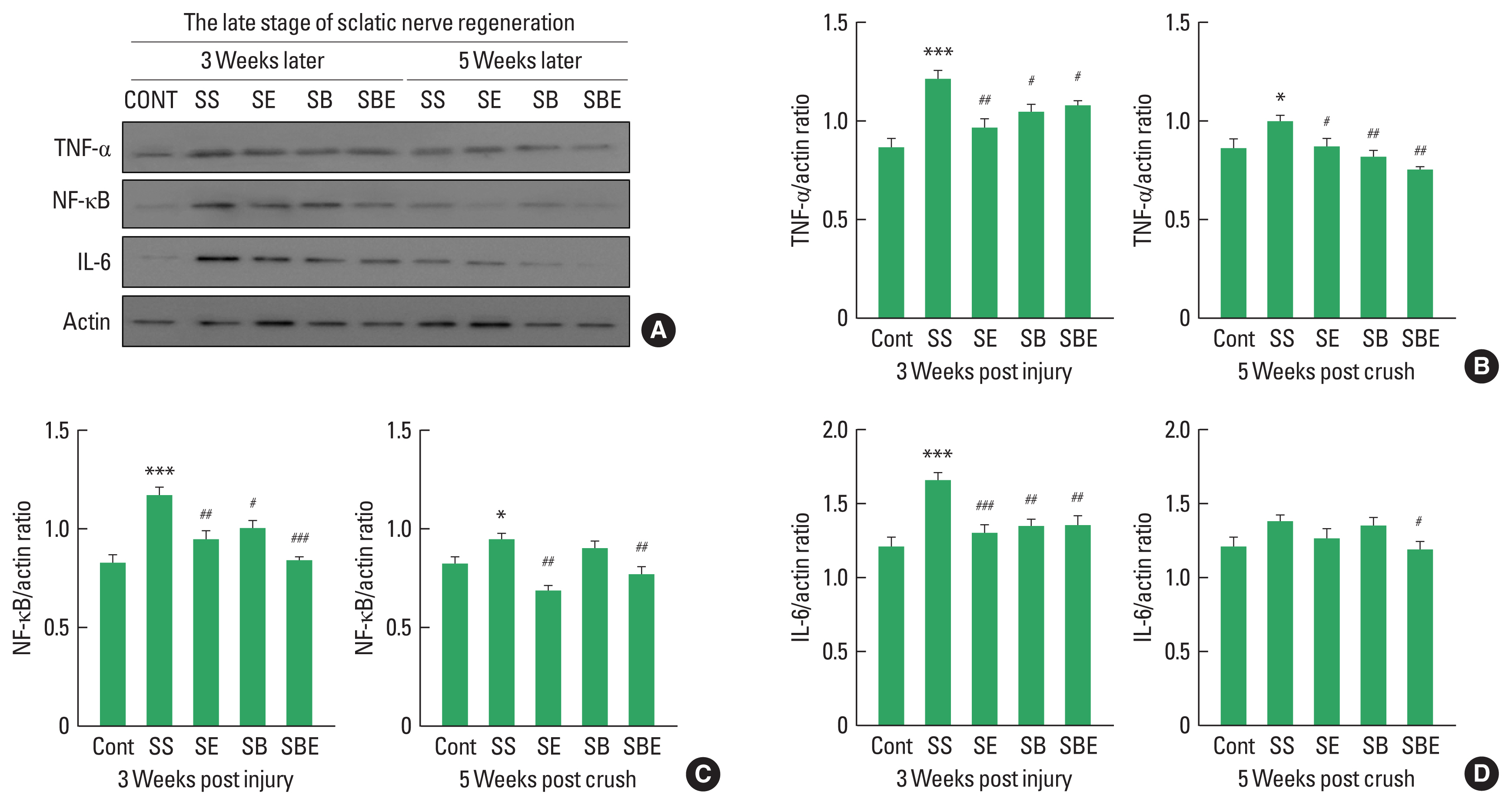
Pain-related molecules in DRG at late stages of regeneration
Increased proinflammatory cytokines in late stage of regeneration may disturb restoration of extracellular matrix, axonal regrowth, and pain relief in the injured sciatic nerve and DRG (Pan et al., 2009). As shown in Fig. 4, expression levels of NF-κB, and TNF-α maintained statistically high in SS group until week 5 postinjury (Fig. 4B, C), whereas SE and SBE groups considerably downregulated TNF-α and NF-κB except for IL-6 at late stage of sciatic nerve regeneration (Fig. 4B–D). In particular, SBE group significantly decreased activities of all proinflammatory cytokines up to week 5 after SNI.
DISCUSSION
During sciatic nerve regeneration, the relatively slow rate of axonal regrowth and pain relief are limitations of spontaneous regeneration, and various therapeutic methods including low-intensity exercise and BMSC transplantation have been proposed to overcome these limitation. However, previous studies reported to date have only suggested single intervention effect rather than combined effect of exercise and cell transplantation. Therefore, our study tried to find out the mechanical effects of combined aerobic exercise and BMSC transplantation on positive alteration in nerve fiber regrowth and neuropathic pain-related proteins after SNI.
In a rat model with SNI, BDNF binding to TrkB receptor stimulates axonal sprouting in the early period of sciatic nerve regeneration, and promotes remyelination of the injured sciatic nerves in the late stage (Zhang et al., 2000). In addition, CNTF has been known to be mediator to promote a number of regrowing axon in distal stump of the injury site after SNI (Haggiag et al., 2001). In the present study, we confirmed that treadmill exercise alone increased neurotrophic factors such as TrkB, BDNF, and CNTF in the early stage of nerve regeneration, but combination of exercise and BMSC transplantation continuously kept up them until the late stage. These findings means that concurrent intervention of exercise and cell transplantation during nerve regeneration may be a stimulator to increase the rate of axonal elongation in the injured sciatic nerve. Previous studies on role of neurotrophic factors in the injured sciatic nerve suggested faithful evidence that combined treatment with BDNF and CNTF improved axonal outgrowth and remyelination (Lang et al., 2011; Zhao et al., 2013), and that low-intensity aerobic exercise increased axon diameter of the injured sciatic nerve through facilitation of Schwann cell expressing BDNF and CNTF (Bobinski et al., 2011). In addition, Seo et al. (2021) confirmed that combined approaches of exercise and BMSC transplantation accelerated BDNF expression level until 28 days after SNI to promote sciatic nerve regeneration. These previous results on activation in BDNF and CNTF over time in the injured sciatic nerve support our findings that combined application might continuously maintain induction levels of neurotrophic factors for enhancing the rate of axonal elongation until week 5 after SNI.
SNI results in dynamin changes in proinflammatory cytokines including NFκB, IL-6, and TNF-α in the injured sciatic nerve and DRG at different timepoint during regeneration (Zhang et al., 2020), and these cytokines have been reported to be highly associated with inflammation and neuropathic pain after peripheral nerve injury (Meunier et al., 2007). For this reason, we investigated pain recovery mechanism of exercise intervention and BMSC transplantation on proinflammatory cytokines over time in ipsilateral L4–5 DRG after SNI. Looking at our results, NF-κB, IL-6, and TNF-α were progressively expressed in the ipsilateral DRG up to the late stage of nerve regeneration, but exercise alone application and/or BMSC transplantation meaningfully decreased activation of three proinflammatory cytokines at each timepoint during sciatic nerve regeneration. What is particular important is that the combination of exercise and transplantation more effectively reduced levels of pain-related cytokines than exercise alone. Several previous studies reported that IL-6 and TNF-α in ipsilateral DRG were upregulated from 1 hr to 7 days after SNI when the pain was most severe, and they is key regulator for identifying neuropathic pain (Uçeyler et al., 2007), and that lentiviral-mediated NF-κB blockade in DRG attenuated SNI-induced neuropathic pain and hyperalgesia (Tegeder et al., 2004). In addition, regular exercise for 14 days after SNI and BMSC transplantation treatment ameliorated activation of inflammatory parameters in L4–5 DRG (Cho and Seo, 2022) and pain behavior (Schäfer et al., 2014). These previous and present results implicate that the combined intervention of treadmill exercise and BMSC transplantation might be one of the effective treatment strategies for recovering SNI-induced neuropathic pain.