AbstractThis study aimed to investigate the effect of whole body vibration (WBV) on the sensory and motor nerve components with sciatic nerve injury model rats. Surgery was performed on 21 female Wister rats (6–8 weeks) under intraperitoneal anesthesia. The nerve-crush injuries for the left sciatic nerve were inflicted using a Sugita aneurysm clip. The sciatic nerve model rats were randomly divided into two groups (n=9; control group, n=12; WBV group). The rats in the WBV group walked in the cage with a vibratory stimulus (frequency 50 Hz, 20 min/day, 5 times/wk), while those in the control group walked in the cage without any vibratory stimulus. We used heat stimulation-induced sensory threshold and lumbar magnetic stimulation-induced motor-evoked potentials (MEPs) to measure the sensory and motor nerve components, respectively. Further, morphological measurements, bilateral hind-limb dimension, bilateral gastrocnemius dimension, and weight were evaluated. Consequently, there were no significant differences in the sensory threshold at the injury side between the control and WBV groups. However, at 4 and 6 weeks postoperatively, MEPs latencies in the WBV group were significantly shorter than those in the control group. Furthermore, both sides of the hind-limb dimension at 6 weeks postoperatively, the left side of the gastrocnemius dimension, and both sides of the gastrocnemius weight significantly increased. In conclusion, WBV especially accelerates the functional recovery of motor nerve components in sciatic nerve-crush injury model rats.
INTRODUCTIONThe peripheral nerve has two primary physiological functions. The first is transmitting sensory information from the sensory organ to the central nervous system, and the second is transmitting the motor information from the spinal cord α-motoneuron to the neuromuscular junction and muscle (Maugeri et al., 2021). The former is called ascending tract or sensory nerve; the latter is called descending tract or motor nerve (Maugeri et al., 2021). In these functions, the motor nerve is vital, and dysfunction of the motor nerve, such as injury, causes decreased muscle power and muscle atrophy, directly affecting activity of daily living and quality of life (Kim et al., 2011; Noble et al., 1998).
The treatments for peripheral nerve injury depend on the severity (Noble et al., 1998; Pabari et al., 2014). For mild injury, conservative ways, including therapeutic exercise, physical therapy, bracing, and electrostimulation, are selected (Gordon and English, 2016; Schiemanck et al., 2015). For severe cases with nerve defects, primary nerve repairs, autologous nerve graft, artificial nerve graft, and nerve transfer surgery are performed (Noble et al., 1998; Pabari et al., 2014).
Recently, a vibratory stimulus has also been conducted as a therapeutic exercise (Costantino et al., 2014; Fattorini et al., 2021; Tahir et al., 2022; Yang et al., 2022). The vibration stimulus is divided into two types of stimuli: focal vibration (Fattorini et al., 2021; Tahir et al., 2022) and whole body vibration (WBV) (Costantino et al., 2014; Yang et al., 2022). Both vibration types affect blood flow (Betik et al., 2021; Tahir et al., 2022), stretching (Kurt, 2015), and pain relief (Hansson and Ekblom, 1986; Rittweger et al., 2002; Wu et al., 2022), and additionally, the WBV induces muscle contraction (Berner et al., 2020; Jo et al., 2021). In addition, the beneficial nerve effects on both types of vibration are already published in animal or human research. For example, local vibration affects sensory thresholds (Doi et al., 2018), H-reflex (Souron et al., 2019), motor-unit (Thompson et al., 2022), and recovery of nerve function (Yin et al., 2022). WBV modulates spinal reflex (Kipp et al., 2011; Sayenko et al., 2010), α-motor neuron excitability (Hortobágyi et al., 2014), and growth hormone concentration (Paineiras-Domingos et al., 2017). Moreover, local vibration for extensor carpi radialis (ECR) muscle reportedly modulates the transcranial magnetic stimulation-induced motor-evoked potentials (MEPs) of the ECR (Rosenkranz et al., 2003).
The peripheral nerve injury model animals have been utilized with sciatic nerve, and the injury is made with compression of a surgical clip (Iwatsuki et al., 2013), forceps (Hamad et al., 2022), surgical hemostats (Wang et al., 2018), and a Dead-weight machine (Mazzer et al., 2008). The model animal research are evaluated with compound action potentials (Hamad et al., 2022; Lin et al., 2018), behavior (Alvites et al., 2021), gait analysis (Alvites et al., 2021; Wang et al., 2018), histological evaluation (Alvites et al., 2021; Wang et al., 2018). Further, we also investigated the impact of the nerve wrapping with both sensory thresholds and lumbar magnetic stimulation (LMS)-induced MEPs (Sonohata et al., 2023).
More recently, the noneffect of WBV therapy for sciatic nerve injury was published (de Oliveira Marques et al., 2021). They evaluated the effects of the WBV with both behavior tests and morphometric analysis of the sciatic nerve (de Oliveira Marques et al., 2021). However, they do not still explore the influence of WBV therapy using functional evaluation of motor and sensory components in the sciatic nerve.
Therefore, this study aimed to investigate the effect of WBV with functional evaluation, such as LMS-induced MEPs and sensory threshold, for sciatic nerve injury model rats.
MATERIALS AND METHODSAnimals and surgeryTwenty-one female Wister rats (6–7 weeks old, weighing approximately 200 g) were were housed in a controlled laboratory environment at a range of temperature (22°C±2°C) in 50%±10% humidity and under a 12-hr light/dark cycle. Standard lab chow and water were provided ad libitum throughout the experiment. All experimental protocol and animal maintenance procedures were approved by the Animal Research Committee of Kumamoto Health Science University (Permit number: animal 19–12). The protocols were per the guidelines of the Animal Protection and Management Law of Japan and the Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983).
Animal surgery was performed under intraperitoneal anesthesia using a mixture of anesthetic agents (0.15 mg/kg medetomidine, 2 mg/kg midazolam, and 2.5 mg/kg butorphanol) after inhalation anesthesia with isoflurane (Kawai et al., 2011). The left lateral femoral region was cleaned with an antiseptic solution, and surgery was performed using a scalpel. A unilateral muscular incision was made from the greater trochanter to the mid-femur, exposing the sciatic nerve. All left sciatic nerves were dissected from the surrounding tissue, and nerve-crush injuries were inflicted using a Sugita aneurysm clip (17-001-02, Mizuho Medical Innovation, Tokyo, Japan) (Iwatsuki et al., 2013). The clip was applied for 5 min with approximately 1.5 N of holding force (Fig. 1A) (Iwatsuki et al., 2013). The skin was then closed with a 4-0 nylon suture. After the surgery was finished for each rat, atipamezole (1.5 mg/kg) was injected intraperitoneally. Subsequently, the rats recovered; each rat was returned to cages for bleeding in the animal room. Although five rats were bred in a rat’s cage preoperatively, each rat was separately bred in a mouse’s cage postoperatively. In the cage, two hand warmers were put under the two-layer pet-sheet to keep the rat body temperature warm.
Measurement of the sensory threshold with heat stimulusThe sensory threshold was measured using heat stimulation before and 2–8 weeks postoperatively (Doi et al., 2018; Nakata et al., 2020). Briefly, an awake rat was immobilized in a plastic tube. A probe with a 25 mm×25 mm surface was placed on the plantar surface of the left hind foot to measure the sensory threshold, and heat stimuli (Intercross-210, Intercross Inc., Tokyo, Japan) were applied to the plantar surface, with the rat in a prone position. The Intercross-210 was connected to a personal computer. We measured the time from the onset of heat stimulation to the observation of the withdrawal reflex (Sonohata et al., 2023).
Measurement of LMS-induced MEPsEach rat was initially anesthetized with a mixture of agents (0.15-mg/kg medetomidine, 2-mg/kg midazolam, and 2.5-mg/kg butorphanol). Subsequently, hair in the lower body of rats was removed with hair clippers, and each rat was fixed with stereotaxic apparatus (Narishige, Tokyo, Japan); their trunks were elevated with mini-jack (10 cm×10 cm, MonotaRO, Amagasaki, Japan) until both the head and trunk become in a horizontal position. Under these conditions, a double-corn coil (4610, Magstim Co. Ltd., Spring Garden, UK), connected with a magnetic stimulator (M200, Magstim Co. Ltd.), was put on the rat’s lumbar region. A single magnetic stimulation was imposed on the rats, and MEPs were recorded into lab chart 8 software via power lab system (AD Instruments, Dunedin, New Zealand). We used magnetic stimulation on the lumbar region (LMS)-induced MEPs to measure motor nerve responses (Sonohata et al., 2023). To simultaneously record bilateral responses from both gastrocnemius muscles, two- needle electrodes for non-references (MLA1203, needle electrode, AD Instruments) were inserted into the middle portion of the lateral head at the bilateral gastrocnemius. Both sides of the reference electrodes (EM-275S, Noraxon, Arizona, AZ, USA) were attached to the planter surface of their bilateral feet. Two ground electrodes (EM-275S, Noraxon) were put on the bilateral pelvic area. The low-cut filter for the recording was 300 Hz, and the high-cut was 500 Hz (Sonohata et al., 2023). After measuring the MEPs, atipamezole (1.5 mg/kg) was injected intraperitoneally. We returned each rat to its cage in the animal room. To maintain each rat’s body temperature after MEPs experiments, we used the hand warmers and the two-layer pet-sheet in each cage.
Analysis of LMS-induced MEPsRecorded bilateral MEP responses from gastrocnemius muscle had three characteristics (Fig. 1Bi). The first was stable and symmetrical (Sonohata et al., 2023), the second was intensity dependency for the LMS, and the third was composed of 1st negative wave, 2nd positive wave, and 3rd small negative (Fig. 1Bi) (Sonohata et al., 2023). Here, the latencies and amplitudes of these three waves were analyzed using lab chart reader software (AD Instruments). The latencies were measured at the points of first wave basements (right latency 1 base, RL1 base; left latency base, LL1 base), first wave tops (right latency 1 top, RL1 top; left latency 1 top, LL1 top), second-wave base (RL2 base and LL2 base), second-wave top (RL2 top and LL2 top), third wave base (RL3 base and LL3 base), and third wave top (RL3 top and LL3 top) (Fig. 1Bi). The amplitudes were measured at RA1 (=RL1 top–RL1 base), RA2 (=RL1 top–RL2 base), RA3 (=RL2 top–RL2 base), RA4 (RL2 top–RL3 base), LA1 (=LL1 top–LL1 base), LA2 (=LL1 top–LL2 base), LA3 (=LL2 top–LL2 base), and LA4 (LL2 top–LL3 base) (Fig. 1Bii). We showed only LA1, LA2, LA3, and LA4 (Fig. 1Bii). Here, although we analyzed bilateral MEP latencies and amplitudes recorded before sciatic nerve surgery, bilateral gastrocnemius muscle MEPs were not statistically different at latencies and amplitudes before surgery (Supplementary Table 1).
Measurement of hind-limb dimension, weight of the gastrocnemius muscle, and gastrocnemius dimensionUnder the condition of removing the hair on the rat’s lower body with hair clippers, both left and right hind-limb pictures were taken from the above side on the prone position with a digital camera (EX-XR3000, Casio, Tokyo, Japan) at every 2 weeks. As a analytical way, under the opening of pictures (JPEG format) with ImageJ software, we selected the freehand line tool in the ImageJ, and the “pixel” on the screen was exchanged to “cm” with the set scale tool in the ImageJ. Then, region of each hind limb was surrounded with the freehand line tool, and the hind-limb’s dimentions were calculated automatically with measure function in the ImageJ (Supplementary Fig. 1A). Eight weeks postoperatively, all rats were sacrificed, and gastrocnemius muscles on both sides were isolated. Subsequently, the muscle weight was determined using a mass scale, and the dimensions of the gastrocnemius were calculated using the ImageJ as we mentioned above (Supplementary Fig. 1B).
WBV and experimental protocolAfter the sciatic nerve operation, the rats were randomly classified into two groups as follows: the control (n=9) and WBV groups (n=12) (Fig. 1C). Different interventions were conducted on the mice in these two groups over the 8-week duration. The rats of both groups were put in the rat cage (CL-0143, 355 mm×499 mm×198 mm, CLEA Japan, Inc., Tokyo, Japan) on the WBV apparatus (Power Plate, Northbrook, IL, USA) separately. Then, another rat cage (CL-0143, 355 mm×499 mm×198 mm) was stacked on the base cage (Supplementary Fig. 2). This way can avoid the standing position of rats. Subsequently, the WBV group’s rats which were put in the stacked cage gave a vibratory stimulus (50 Hz, 5 min×4 sets=20 min/day, 5 times/wk). On the contrary, the control group’s rats only put in the stacked cage without any vibratory stimulus (5 min×4 sets=20 min/day, 5 times/wk) (Supplementary Fig. 2).
Statistical analysisExperimental data are expressed as mean±standard deviation. Single comparisons were conducted using Wilcoxon signed-rank test for paired groups and the Mann–Whitney U-test for unpaired groups. P<0.05 was considered statistically significant (In each figure, “*” was shown as “significant”, and “ns” or “no mark” was present as “not significant”). Subsequently, Kaplan–Meier survival curves were calculated. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Moreover, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (Kanda, 2013).
RESULTSThe effects of WBV on heat-stimulation-induced sensory thresholdThere were no significant differences in the sensory threshold at the injury side between the control and WBV groups (before surgery; 53.2°C±1.13°C vs. 53.3°C±0.93°C, 2 weeks postoperatively; 53.5°C±2.09°C vs. 54.18°C±2.10°C, 4 weeks postoperatively; 53.0°C±1.62°C vs. 53.71°C±1.64°C, 6 weeks postoperatively; 52.53°C±1.09°C vs. 53.17°C±1.30°C, 8 weeks postoperatively; 53.82°C±1.59°C vs. 52.90°C±0.86°C) (Fig. 2).
The effects of WBV for both LMS-induced MEPs amplitude and latency at 2 weeks postoperativelyWe compared LMS-induced MEP amplitudes and latencies between the control and WBV groups 2 weeks postoperatively. There were no significant differences in the amplitude parameters between the two groups (Supplementary Fig. 3, n=9 and 12). Furthermore, there were no significant differences in the MEPs latencies (Supplementary Fig. 4, n=9 and 12).
The effects of WBV for both MEPs amplitudes and latencies at 4 weeks postoperativelyWe compared LMS-induced MEPs amplitudes and latencies 4 weeks postoperatively between the control and WBV groups (Figs. 3, 4). There were no significant differences in the amplitude parameters between the two groups (Fig. 3, n=9 and 11). However, regarding MEP latencies, LL1 top, LL1 top–LL1 base and LL1 top–RL1 top were significantly different between the two groups (LL1 top; 5.56±0.43 msec vs. 4.59±0.59 msec, *P=0.0016, LL1 top–LL1 base; 2.38±0.45 msec vs. 1.42±0.55 msec, *P= 0.0013, data not shown, LL1 top–RL1 top; 1.50±0.48 msec vs. 0.70±0.47 msec, *P=0.0067) (Fig. 4Bii, Biv).
The effects of WBV for both MEPs amplitudes and latencies at 6 weeks postoperativelySix weeks postoperatively, we compared LMS-induced MEP amplitudes and latencies between the control and WBV groups (Figs. 5, 6). There were no significant differences in amplitude parameters between the two groups (Fig. 5B, n=7 and 10). Furthermore, regarding latencies, LL1 top–RL1 top, LL3 top, and LL3 base–RL3 base in the WBV group were significantly shorter than that in the control group (LL1 top–RL1 top; 1.17±0.26 ms vs. 0.52±0.37 ms, *P=0.0033, LL3 top; 12.06±1.53 ms vs. 10.32± 0.95 ms, *P=0.0031, LL3 base–RL3 base; 1.57±1.38 ms vs. 1.02±1.54 ms, *P=0.027) (Fig. 6Biv, Bx, and Bxi).
The effects of WBV for both MEPs amplitudes and latencies at 8 weeks postoperativelyWe compared LMS-induced MEP amplitudes and latencies between the control and WBV groups (Figs. 7, 8) 8 weeks postoperatively. LA1, LA2 and LA3 amplitudes were significantly different between the two groups (LA1; 0.53±0.19 vs. 0.82±0.11, *P= 0.004, LA2; 0.81±0.35 vs. 1.41±0.33, *P=0.004, LA3; 1.32± 0.60 vs. 2.19±0.61, *P=0.02, LA4; 0.26±0.29 vs. 0.33±0.12, ns: not significant, P=0.142) (Fig. 7Bi–Biii). However, in the latencies, LL1-, LL2-, and LL3-related parameters did not affect the two groups (Fig. 8B).
The effects of WBV for bilateral hind-limb dimensionHind-limb dimension at the injured left side was significantly increased only 6 weeks postoperatively in the control and WBV groups (6.56±0.57 cm2 vs. 7.49±0.71 cm2, *P=0.019) (Fig. 9Bi). Furthermore, the hind-limb dimension on the noninjured right side was significantly increased only 6 weeks postoperatively in the control and WBV groups (7.14±0.60 cm2 vs. 8.00±0.60 cm2, *P=0.014) (Fig. 9Bii).
The effects of WBV for both dimension and weight of gastrocnemius muscleGastrocnemius dimension was significantly increased only at the left side of the injury in the control and WBV groups (2.57± 0.17 cm2 vs. 2.92±0.14 cm2, *P=0.0087) (Fig. 10Bi) but not at the noninjured right side (2.85±0.24 cm2 vs. 3.08±0.07 cm2, ns: not significant, P=0.106) (Fig. 10Bii). Furthermore, gastrocnemius weight was significantly increased at both sides in the control and WBV groups (left side; 1.10±0.04 g vs. 1.22±0.09 g, *P=0.0028, right side; 1.28±0.03 g vs. 1.40±0.10 g, *P=0.0035) (Fig. 10Biii, Biv).
DISCUSSIONWe investigated the effect of the WBV on the sensory threshold, LMS-induced MEPs, and morphological evaluation. We found that WBV is more effective for motor nerve components than sensory components, and the effects were observed at 4–8 weeks, particularly between 4 and 6 weeks. Moreover, the axon diameter of sensory fibers, such as touch-related Aβ fibers, fast pain-related Aδ fibers, and slow pain-related C fibers, are reportedly smaller than motor nerves as Aα fibers (Perl, 2007). Therefore, physical clip compression for the sciatic nerve is believed to cause more damage to the motor than the sensory component. This study showed that the WBV improved more damaged motor nerve components.
Whole body vibration and sensory effectsWe suggested reasons for the noneffect of WBV on the sensory component. Our previous study showed that nerve wrapping for compressing injured sciatic nerve only affected the sensory components at 2 weeks postoperatively (Sonohata et al., 2023), suggesting that the sensory-related narrow fibers, such as Aδ or C fibers, might have spontaneous recovery after 2 weeks. In this study, however, even at 2 weeks, there were no significant differences between the control and WBV groups (Fig. 2). Since the vibration seems to activate the Vater-Pacini corpuscle and Aβ fibers (O’Mara et al., 1988) through rapid-adapting type II (RA-II) (Pestell and Lepora, 2022). The RA-II activation is only likely to generate action potentials firing at the initial and terminal phase of the vibration, but not all the phases (Widdicombe, 2003).
Therefore, since the RA-II may not so much generate the action potentials in the sensory components of the sciatic nerve, RA-II-related Vater-Pacini corpuscle and Aβ fibers activation might not drastically impact on the pain threshold associated Aδ or C fibers.
Whole body vibration and motor nerve effectsThe origin of the second (L2) and third (L3) wave components is still unclear (Fig. 6Bx, Bxi) (Sonohata et al., 2023). However, we speculate that these components might not be “artificial noise” because the “artificial noise” of MEPs was suppressed after sciatic nerve injury (Figs. 3Fig. 4Fig. 5Fig. 6Fig. 7–8; Supplementary Figs. 3, 4). Furthermore, since the L2 wave component is very stable, it might not be “a kind of antidromic response, such as F-wave” (Fisher, 2007). However, the neurophysiological meanings of these wave components are still unknown. In this study, therefore, we only discuss the effect of the first (L1) component.
The LMS is believed to stimulate the sacral nerves (Maccabee et al., 1996). Therefore, we suspect that RL1 and LL1 may be Aα fiber-originated components, suggesting that WBV might act on the Aα fiber’s recovery. In addition to the vibration-induced activation of the RA-II mentioned above, LTMR, Pacini corpuscle, and Aβ fiber, WBV may stimulate the palm and sole and the whole body’s skin and skeletal muscles (Rosenkranz et al., 2003). If WBV acts on the skeletal muscle and stretches the muscle, WBV might activate the muscle spindle (Giszter and Kargo, 2002; Pope and DeFreitas, 2015). Consequently, activating Ia afferent fiber in the muscle spindle mono-synaptically transmits spinal α-motoneuron (Pope and DeFreitas, 2015). The excitability of the α-motoneuron is believed to contract the muscle via Aα fiber. This vibration-induced reflex is called the tonic vibration reflex (TVR) (Bonanni et al., 2022). Therefore, the vibration-induced activation of TVR may facilitate the recovery of Aα fiber-associated motor component of the sciatic nerve (Figs. 3Fig. 4Fig. 5Fig. 6Fig. 7–8).
In this study, MEPs differences between the control and WBV groups were observed at 4–8 weeks. Moreover, the MEPs latencies and amplitudes were changed at 4–6 weeks (Figs. 4B, 6B) and 8 weeks (Fig. 7B), respectively. In clinical studies with transcranial magnetic stimulation-induced MEPs responses, MEPs latencies are more useful as the evaluation parameter for nerves than amplitude because the central nervous system, including MEPs amplitude, often fluctuates (Giambattistelli et al., 2014). However, since the simultaneous bilateral MEPs are very stable (Sonohata et al., 2023), latencies and amplitudes may adopt as the parameter for the evaluation.
We hypothesized as follows regarding the recovery of MEPs latencies and amplitudes. First, if some single motor nerves in the injured sciatic nerve were partially recovered in the wrapping group, LL1 base and LL1 top latencies may appear (Figs. 4, 6). Second, the more the single motor nerves are recovered, the more the amplitude of the LL1 component might increase (Fig. 7).
Whole body vibration and morphological effectsThe significant increase in hind-limb dimension (Fig. 9Bi, Bii) and gastrocnemius weight (Fig. 10Biii, Biv) may result from two effects. One is TVR-induced Aα fiber facilitation and indirect skeletal muscle contraction; the other may be the direct facilitation of muscle contraction caused by WBV. Further, that WBV significantly increased not only the injury side (left) but also the right hind-limb’s dimension and right gastrocnemius’s weight was unexpected (Figs. 9Bii, 10Biv). However, there were no statistical differences in the dimension of the right gastrocnemius (Fig. 10Bii). This may have resulted from a limitation of two-dimensional analysis with ImageJ, which only measures the x- and y-axis but not the z-axis; that is, the measurement cannot reflect the gastrocnemius’s thickness.
In this study, the differences in morphology between the control and WBV groups corresponded to the results of MEPs at 6 and 8 weeks postoperatively, suggesting that the recovery of the motor component in the sciatic nerve (MEPs) may have increased the hind-limb volume and muscle weight.
Research limitationRecently, the noneffect of WBV therapy for sciatic nerve injury was published (de Oliveira Marques et al., 2021). They used WBV training (15 min/day, 5 times/wk, and 5 weeks) and evaluated with behavior tests (the Sciciatic-Functional Index, the Horizontal Ladder Rung Walking Test, and the Narrow Beam Test) and morphometric analysis of the sciatic nerve at 2–5 weeks postoperatively (de Oliveira Marques et al., 2021). Although there are many methodological differences between our study and theirs, the most critical discrepancy may be the follow-up period. In addition, in our study, there were no statistical differences between the control and WBV groups until 2 weeks after the injury (Supplementary Figs. 3, 4). However, the discrepancy among morphometric analysis, electrophysiological measurement, and behavior tests should be better discussed.
A limitation of the study might be the effect of the anesthesia used for the LMS-induced MEPs experiments. In the study, we used a mixture of agents (medetomidine, midazolam, and butorphanol) and a recovery agent (atipamezole) for each rat (Kawai et al., 2011). Furthermore, to maintain the body temperature of each rat during and after surgery, we utilized the hand warmers and the two-layer pet-sheet (see method). However, rats suddenly died in a cage (Supplementary Fig. 5A). Almost all rats died the day they injected agents after LMS-induced MEPs experiments were concluded (inserted pie graph in Supplementary Fig. 5B). Moreover, the increased frequency of injected agents may be related to the death of rats (Supplementary Fig. 5B). This is the reason for the dispersion of numbers in each figure. Therefore, we are trying to perform the MEPs measurements under awake but not anesthetized conditions.
Clinical applicationTherapeutic exercises generally point to dynamic exercises, such as walking, jogging, muscle strength, and bike ergometer (Barker and Eickmeyer, 2020). However, these exercises are not always applied to patients with orthopedic or peripheral nerve problems. Furthermore, these patients cannot always do such dynamic exercises in the acute stage postoperatively. In such cases, if WBV can be applied to patients in the sitting or supine positions, injury recovery at both motor nerve and skeletal muscle might be facilitated even though classical dynamic exercises are not performed (Berner et al., 2020; Jo et al., 2021; Yin et al., 2022).
In conclusion, we investigated the effect of WBV on nerve injury model rats by measuring motor and sensory components. Although the sensory nerve component did not have any effects on WBV, the addition of WBV statistically accelerated the functional recovery of MEPs as the motor nerve component. Furthermore, WBV increased the bilateral side of the hind-limb dimension and gastrocnemius’s weight.
SUPPLEMENTARY MATERIALSSupplementary Figs. 1–5 can be found via https://doi.org/10.12965/jer.2346178.089.
Supplemental Table 1.Averaged amplitude and latency of lumbar magnetic stimulation-induced motor-evoked potentials from bilateral gastrocnemius muscle before surgery
Supplementary Fig. 1.An analytical way of dimension measurements at both bilateral hind-limb and gastrocnemius. (A) A example of region to measure right hind-limb dimension (yellow line and blue arrows). (B) A example of region to measure both sides of gastrocnemius muscle (yellow lines). Lt., left; Rt., right.
Supplementary Fig. 2.Division of two groups, and WBV protocols (see methods). WBV, whole body vibration.
Supplementary Fig. 3.Lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) amplitudes at 2 weeks postoperatively. (A) An example of a single bilateral MEPs raw data 2 weeks after sciatic nerve surgery. (Ai) Control group. (Upper panel of Ai) LMS-induced MEPs from the right gastrocnemius muscle (Rt. gastro. M). (Lower panel of Ai) LMS-induced MEPs from left gastrocnemius muscle (Lt. gastro. M). (Aii) WBV group. (Upper panel of Aii) LMS-induced MEPs from Rt. gastro. M. (Lower panel of Aii) LMS-induced MEPs from Lt. gastro. M. (B) Bar graphs of amplitude related to four parameters and comparison between the control group (left bar, n=9) and WBV group (right bar, n=12). (Bi) Comparison of LA1. (Bii) Comparison of LA2. (Biii) Comparison of LA3. (Biv) Comparison of LA4. RA1, the amplitude value of (RL1 top–RL1 base); RA2, the amplitude value of (RL1 top–RL2 base); RA3, the amplitude value of (RL2 top–RL2 base); LA4, the amplitude value of (RL2 top–RL3 base); LA1, the amplitude value of (LL1 top–LL1 base); LA2, the amplitude value of (LL1 top–LL2 base); LA3, the amplitude value of (LL2 top–LL2 base); LA4, the amplitude value of (LL2 top–LL3 base); WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant.
Supplementary Fig. 4.Lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) latencies at 2 weeks postoperatively. (A) An example of a single left MEPs raw data 2 weeks after sciatic nerve surgery. (Upper panel of A) Control group. (Lower panel of A) WBV group. (B) Bar graphs of latencies related to twelve parameters and comparison between the control group (left bar, n=9) and WBV group (right bar, n=12). (Bi) Comparison of LL1 base. (Bii) Comparison of LL1 top. (Biii) Comparison of LL1 base – RL1 base. (Biv) Comparison of LL1 top – RL1 top. (Bv) Comparison of LL2 base. (Bvi) Comparison of LL2 top. (Bvii) Comparison of LL2 base – RL2 base. (Bviii) Comparison of LL1 top – RL1 top. (Bix) Comparison of LL3 base. (Bx) Comparison of LL3 top. (Bxi) Comparison of LL3 base – RL3 base. (Bxii) Comparison of LL3 top – RL3 top. RL1, right side’s latency 1; RL2, right side’s latency 2; RL3, right side’s latency 3; LL1, left side’s latency 1; LL2, left side’s latency 2; LL3, left side’s latency 3; WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant.
Supplementary Fig. 5.Survival analysis of animals in this study. (A) The Kaplan–Meier survival analysis of between control and WBV groups. (inserted bar grph in A) Mortality rate of both control and WBV groups at 70 days after surgery. (B) Relationship between numbers of drug injection for anesthetization and numbers of death. (inserted pie grph in B) The day which rats died after the injection of the mixed agents and atipamezole. WBV, whole body vibration.
ACKNOWLEDGMENTSWe thank Dr. Hiroharu Koga (Kumamoto Health Science University), Mr. Yasuyuki Teramoto (Kumamoto Kinoh Hospital), Mr. Tsukasa Kajihara (Miyuki Giken Kyusyu Co.), Dr. Kazuyuki Oda (Inrercross Co.), Mr. Soma Yamamoto (Bio Research Center Co. Ltd.) and Mr. Kazuyoshi Morita (Bio Research Center Co. Ltd.) for helpful technical advice for both MEPs and sensory threshold experiments. This work is supported by both JPJS KAKENHI (Grant Numbers 26350646 (AD), 19K11383 (AD)) and Kumamoto Health Science University’s special fellowship (grant number 2020-C-02 (AD)).
REFERENCESAlvites RD, Branquinho MV, Sousa AC, Amorim I, Magalhães R, João F, Almeida D, Amado S, Prada J, Pires I, Zen F, Raimondo S, Luís AL, Geuna S, Varejão ASP, Maurício AC. Combined use of chitosan and olfactory mucosa mesenchymal stem/stromal cells to promote peripheral nerve regeneration in vivo. Stem Cells Int. 2021;2021:6613029
Berner K, Albertyn SCS, Dawnarain S, Hendricks LJ, Johnson J, Landman A, Burger M. The effectiveness of combined lower limb strengthening and whole-body vibration, compared to strengthening alone, for improving patient-centred outcomes in adults with COPD: a systematic review. S Afr J Physiother. 2020;76:1412
Betik AC, Parker L, Kaur G, Wadley GD, Keske MA. Whole-body vibration stimulates microvascular blood flow in skeletal muscle. Med Sci Sports Exerc. 2021;53:375–383.
Bonanni R, Cariati I, Romagnoli C, D’Arcangelo G, Annino G, Tancredi V. Whole body vibration: a valid alternative strategy to exercise? J Funct Morphol Kinesiol. 2022;7:99
Costantino C, Gimigliano R, Olvirri S, Gimigliano F. Whole body vibration in sport: a critical review. J Sports Med Phys Fitness. 2014;54:757–764.
de Oliveira Marques C, Amaro Espindula I, Kwame Karikari Darko E, Viçosa Bonetti L, Sonza A, Aparecida Partata W, Faccioni-Heuser MC, Malysz T. Whole-body vibration therapy does not improve the peripheral nerve regeneration in experimental model. J Musculoskelet Neuronal Interact. 2021;21:68–78.
Doi A, Sakasaki J, Tokunaga C, Sugita F, Kasae S, Nishimura K, Sato Y, Kuratsu T, Hashiguchi S, Shin MC, Yoshimura M. Both ipsilateral and contralateral localized vibratory stimulations modulated pain-related sensory thresholds on the foot in mice and humans. J Pain Res. 2018;11:1645–1657.
Fattorini L, Rodio A, Pettorossi VE, Filippi GM. Is the focal muscle vibration an effective motor conditioning intervention? a systematic review. J Funct Morphol Kinesiol. 2021;6:39
Giambattistelli F, Tomasevic L, Pellegrino G, Porcaro C, Melgari JM, Rossini PM, Tecchio F. The spontaneous fluctuation of the excitability of a single node modulates the internodes connectivity: a TMS-EEG study. Hum Brain Mapp. 2014;35:1740–1749.
Giszter SF, Kargo WJ. Separation and estimation of muscle spindle and tension receptor populations by vibration of the biceps muscle in the frog. Arch Ital Biol. 2002;140:283–294.
Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016;43:336–350.
Hamad MN, Boroda N, Echenique DB, Dieter RA, Amirouche FML, Gonzalez MH, Kerns JM. Compound motor action potentials during a modest nerve crush. Front Cell Neurosci. 2022;16:798203
Hansson P, Ekblom A. Influence of stimulus frequency and probe size on vibration-induced alleviation of acute orofacial pain. Appl Nurophysiol. 1986;9:155–165.
Hortobágyi T, Rider P, DeVita P. Effects of real and sham whole-body mechanical vibration on spinal excitability at rest and during muscle contraction. Scand J Med Sci Sports. 2014;24:e436–447.
Iwatsuki K, Arai T, Ota H, Kato S, Natsume T, Kurimoto S, Yamamoto M, Hirata H. Targeting anti-inflammatory treatment can ameliorate injury-induced neuropathic pain. PLoS One. 2013;8:e57721
Jo NG, Kang SR, Ko MH, Yoon JY, Kim HS, Han KS, Kim GW. Effectiveness of whole-body vibration training to improve muscle strength and physical performance in older adults: prospective, single-blinded, randomized controlled trial. Healthcare (Basel). 2021;9:652
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–458.
Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 2011;60:481–487.
Kim TH, Yoon SJ, Lee WC, Kim JK, Shin J, Lee S, Lee SM. Protective effect of GCSB-5, an herbal preparation, against peripheral nerve injury in rats. J Ethnopharmacol. 2011;136:297–304.
Kipp K, Johnson ST, Doeringer JR, Hoffman MA. Spinal reflex excitability and homosynaptic depression after a bout of whole-body vibration. Muscle Nerve. 2011;43:259–262.
Kurt C. Alternative to traditional stretching methods for flexibility enhancement in well-trained combat athletes: local vibration versus whole-body vibration. Biol Sport. 2015;32:225–233.
Lin T, Qiu S, Yan L, Zhu S, Zheng C, Zhu Q, Liu X. Miconazole enhances nerve regeneration and functional recovery after sciatic nerve crush injury. Muscle Nerve. 2018;57:821–828.
Maccabee PJ, Lipitz ME, Desudchit T, Golub RW, Nitti VW, Bania JP, Willer JA, Cracco RQ, Cadwell J, Hotson GC, Eberle LP, Amassian VE. A new method using neuromagnetic stimulation to measure conduction time within the cauda equina. Electroencephalogr Clin Neurophysiol. 1996;101:153–166.
Maugeri G, D’Agata V, Trovato B, Roggio F, Castorina A, Vecchio M, Di Rosa M, Musumeci G. The role of exercise on peripheral nerve regeneration: from animal model to clinical application. Heliyon. 2021;7:e08281
Mazzer PYCN, Barbieri CH, Mazzer N, Fazan VPS. Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats. J Neurosci Methods. 2008;173:249–258.
Nakata T, Doi A, Uta D, Shin MC, Yoshimura M. Free gait in a shallow pool accelerates recovery after exercise in model mice with fibromyalgia. J Exerc Rehabil. 2020;16:398–409.
Noble J, Munro CA, Prasad VSSV, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122.
O’Mara S, Rowe M, Tarvin R. Neural mechanisms in vibrotactile adaptation. J Neurophysiol. 1988;59:607–622.
Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133:1420–1430.
Paineiras-Domingos LL, Sá-Caputo D, de da C, Moreira-Marconi E, Morel DS, da Fontoura Dionello C, Sousa-Gonçalves CR, Frederico ÉHFF, Marín PJ, Tamini S, Sartorio A, Bernardo-Filho M. Can whole body vibration exercises affect growth hormone concentration? a systematic review. Growth Factors. 2017;35:189–200.
Pestell N, Lepora NF. Artificial SA-I, RA-I and RA-II/vibrotactile afferents for tactile sensing of texture. J R Soc Interface. 2022;19:20210603
Pope ZK, DeFreitas JM. The effects of acute and prolonged muscle vibration on the function of the muscle spindle’s reflex arc. Somatosens Mot Res. 2015;32:254–261.
Rittweger J, Just K, Kautzsch K, Reeg P, Felsenberg D. Treatment of chronic lower back pain with lumbar extension and whole-body vibration exercise: a randomized controlled trial. Spine. 2002;27:1829–1834.
Rosenkranz K, Pesenti A, Paulus W, Tergau F. Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res. 2003;149:9–16.
Sayenko DG, Masani K, Alizadeh-Meghrazi M, Popovic MR, Craven BC. Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci Lett. 2010;482:66–70.
Schiemanck S, Berenpas F, van Swigchem R, van den Munckhof P, de Vries J, Beelen A, Nollet F, Geurts AC. Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restor Neurol Neurosci. 2015;33:795–807.
Sonohata M, Doi A, Uchihashi K, Hashimoto A, Kii S, Inoue T, Mawatari M. Short-term collagen nerve wrapping facilitates motor and sensory recovery from nerve degeneration in a sciatic nerve injury rat model. J Pain Res. 2023;16:1683–1695.
Souron R, Baudry S, Millet GY, Lapole T. Vibration-induced depression in spinal loop excitability revisited. J Physiol. 2019;597:5179–5193.
Tahir S, Baig MO, Rathore FA, Aslam H. The emerging role of focal muscle vibration in rehabilitation of neurological disorders. J Pak Med Assoc. 2022;72:2126–2128.
Thompson CK, Johnson MD, Negro F, Farina D, Heckman CJ. Motor unit discharge patterns in response to focal tendon vibration of the lower limb in cats and humans. Front Integr Neurosci. 2022;16:836757
Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: a comparison between sciatic functional index and kinematic analysis. PLoS One. 2018;13:e0208985
Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A Discov Mol Cell Evol Biol. 2003;270:2–10.
Wu Z, Zou Z, Zhong J, Fu X, Yu L, Wang J, Wang X, Wu Q, Hou X. Effects of whole-body vibration plus hip-knee muscle strengthening training on adult patellofemoral pain syndrome: a randomized controlled trial. Disabil Rehabil. 2022;44:6017–6025.
Yang X, Xue X, Tu H, Li N. Effect of whole-body vibration training on the recovery of lower limb function in people with stroke: a systematic review and meta-analysis. Disabil Rehabil. 2022;Nov. 11. 1–10. 10.1080/09638288.2022.2138993. [Epub].
Fig. 1Sciatic nerve surgery, sensory threshold, and lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) from bilateral gastrocnemius muscle. (A) Surgery for left sciatic nerve. Arrows in (Ai) and (Aii) show the compression sites with the clip. (Ai) A photo of sciatic nerve compression with a Sugita aneurysm clip. (Aii) A photo of sciatic nerve decompression. (B) An example of a single bilateral MEPs raw data before sciatic nerve surgery. (Bi) Measurements of MEPs latency. (Upper panel of Bi) LMS-induced MEPs from the right gastrocnemius muscle. (Lower panel of Bi) LMS-induced MEPs from the left gastrocnemius muscle. (Bii) Y-axis expanded LMS-induced MEPs from the left gastrocnemius muscle and measurements of MEPs amplitude. (C) A flow chart of the experimental protocol. RL1, right side’s latency 1; RL2, right side’s latency 2; RL3, right side’s latency 3; LL1, left side’s latency 1; RL2, left side’s latency 2; RL3, left side’s latency 3; LA1, the amplitude value of (LL1 top–LL1 base); LA2, the amplitude value of (LL1 top–LL2 base); LA3, the amplitude value of (LL2 top–LL2 base); LA4, the amplitude value of (LL2 top–LL3 base); gastro. M, gastrocnemius muscle. 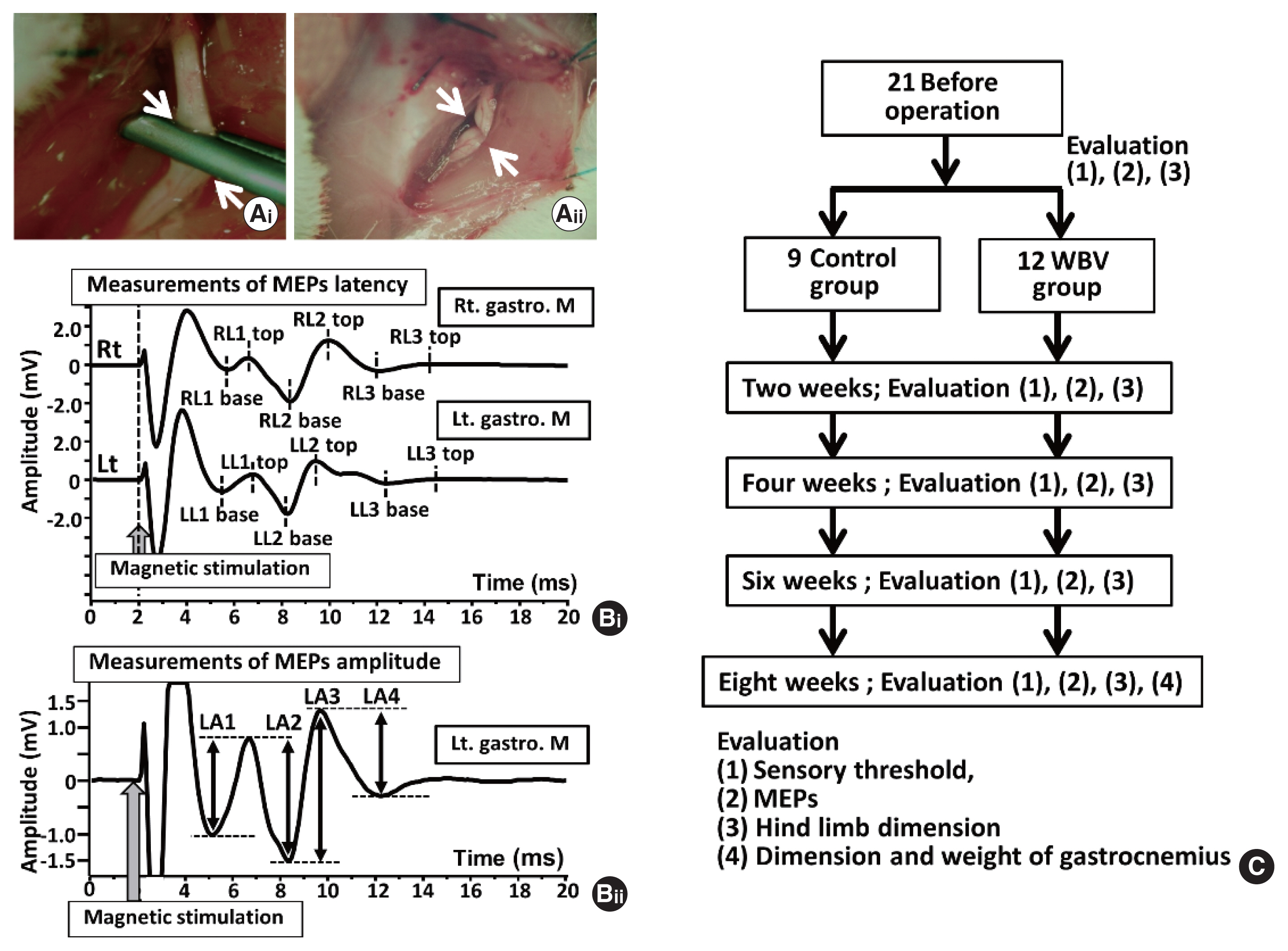
Fig. 2Changing of sensory threshold before and after sciatic nerve-crush model rats between control and whole body vibration (WBV) groups. Lt., left. 
Fig. 3Amplitudes of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) amplitudes at 4 weeks postoperatively. (A) An example of a single bilateral MEPs raw data 4 weeks after sciatic nerve surgery. (Ai) Control group. (Upper panel of Ai) LMS-induced MEPs from right gastrocnemius muscle. (Lower panel of Ai) LMS-induced MEPs from left gastrocnemius muscle. (Aii) WBV group. (Upper panel of Aii) LMS-induced MEPs from right. right gastrocnemius muscle. (Lower panel of Aii) LMS-induced MEPs from left right gastrocnemius muscle. (B) Bar graphs of amplitude related eight parameters and comparison between the control group (left bar, n=9) and WBV group (right bar, n=11). (Bi) Comparison of LA1. (Bii) Comparison of LA2. (Biii) Comparison of LA3. (Biv) Comparison of LA4. RA1, the amplitude value of (RL1 top–RL1 base); RA2, the amplitude value of (RL1 top–RL2 base); RA3, the amplitude value of (RL2 top–RL2 base); LA4, the amplitude value of (RL2 top–RL3 base); LA1, the amplitude value of (LL1 top–LL1 base); LA2, the amplitude value of (LL1 top–LL2 base); LA3, the amplitude value of (LL2 top–LL2 base); LA4, the amplitude value of (LL2 top–LL3 base); WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. 
Fig. 4Latencies of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) latencies at 4 weeks postoperatively. (A) An example of a single left MEPs raw data 4 weeks after sciatic nerve surgery. (Upper panel of 4A) Control group. (Lower panel of 4A) WBV group. (B) Bar graphs of latencies related to twelve parameters and comparison between the control group (left bar, n=9) and WBV group (right bar, n=11). (Bi) Comparison of LL1 base. (Bii) Comparison of LL1 top. (Biii) Comparison of LL1 base – RL1 base. (Biv) Comparison of LL1 top – RL1 top. (Bv) Comparison of LL2 base. (Bvi) Comparison of LL2 top. (Bvii) Comparison of LL2 base – RL2 base. (Bviii) Comparison of LL1 top – RL1 top. (Bix) Comparison of LL3 base. (Bx) Comparison of LL3 top. (Bxi) Comparison of LL3 base – RL3 base. (Bxii) Comparison of LL3 top – RL3 top. RL1, right side’s latency 1; RL2, right side’s latency 2; RL3, right side’s latency 3; LL1, left side’s latency 1; LL2, left side’s latency 2; LL3, left side’s latency 3; WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. *P<0.05. 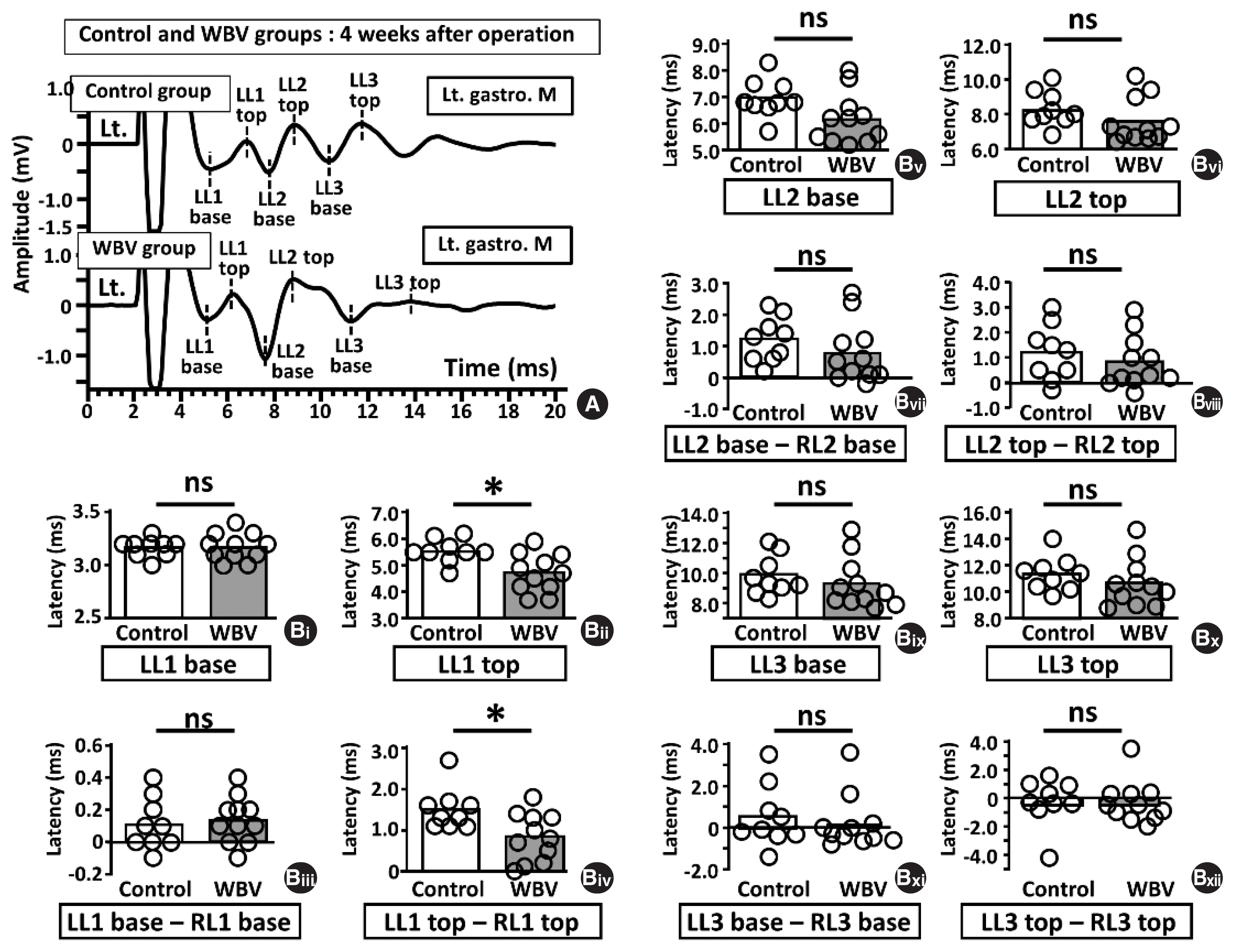
Fig. 5Amplitudes of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) amplitudes at 6 weeks postoperatively. (A) An example of a single bilateral MEPs raw data 6 weeks after sciatic nerve surgery. (Ai) Control group. (Upper panel of Ai) LMS-induced MEPs from Rt. gastro. M. (Lower panel of Ai) LMS-induced MEPs from Lt. gastro. M. (Aii) WBV group. (Upper panel of Aii) LMS-induced MEPs from Rt. gastro. M. (Lower panel of Aii) LMS-induced MEPs from Lt. gastro. M. (B) Bar graphs of amplitude related eight parameters and comparison between the control group (left bar, n=7) and WBV group (right panels, n=10). (Bi) Comparison of LA1. (Bii) Comparison of LA2. (Biii) Comparison of LA3. (Biv) Comparison of LA4. RA1, the amplitude value of (RL1 top–RL1 base); RA2, the amplitude value of (RL1 top–RL2 base); RA3, the amplitude value of (RL2 top–RL2 base); LA4, the amplitude value of (RL2 top–RL3 base); LA1, the amplitude value of (LL1 top–LL1 base); LA2, the amplitude value of (LL1 top–LL2 base); LA3, the amplitude value of (LL2 top–LL2 base); LA4, the amplitude value of (LL2 top–LL3 base); WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. 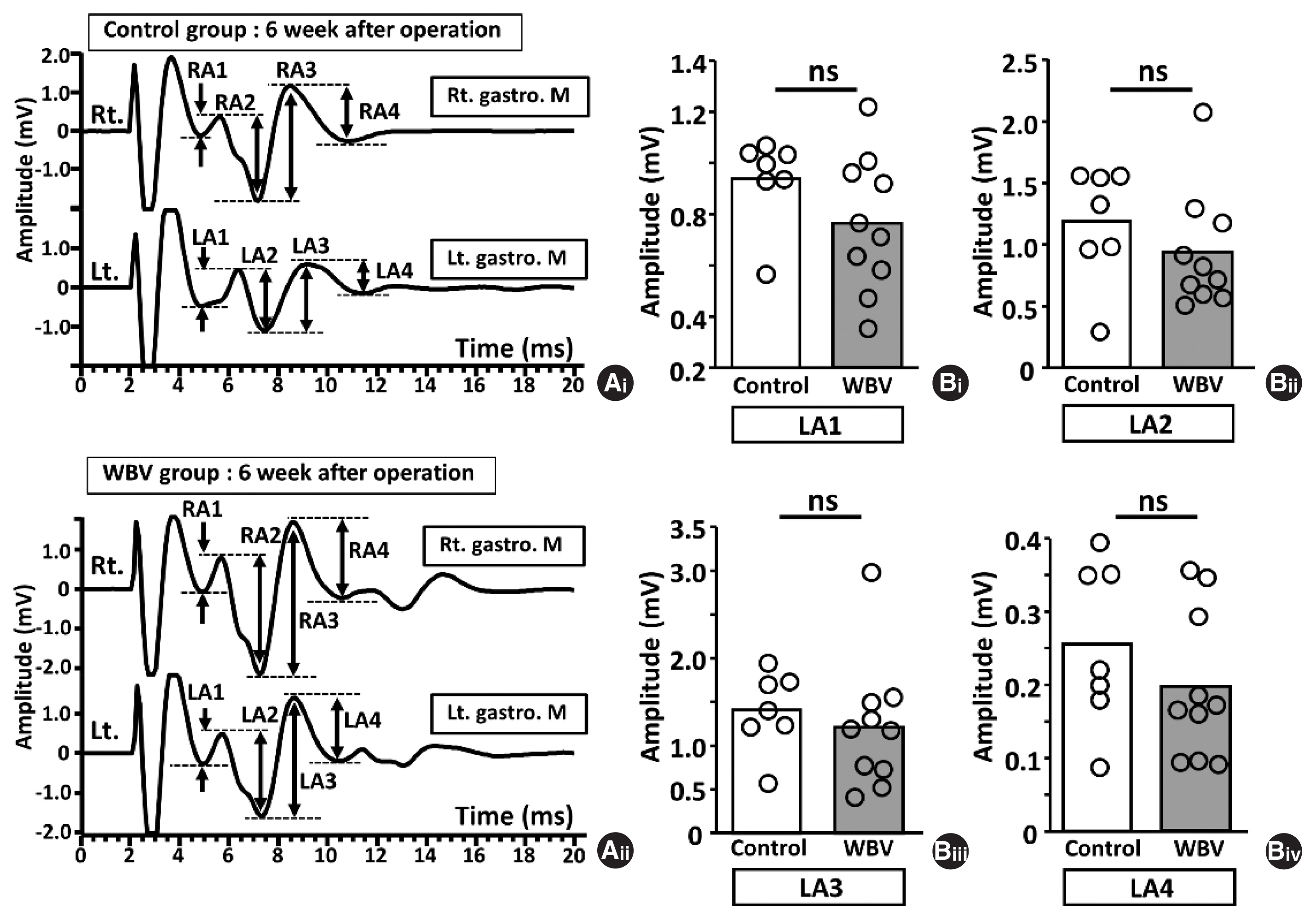
Fig. 6Latencies of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) latencies at 6 weeks postoperatively. (A) An example of a single left MEPs raw data 6 weeks after sciatic nerve surgery. (Ai) Control group. (Aii) WBV group. (B) Bar graphs of latencies related to twelve parameters and comparison between the control group (left bar, n=7) and WBV group (right bar, n=10). (Bi) Comparison of LL1 base. (Bii) Comparison of LL1 top. (Biii) Comparison of LL1 base – RL1 base. (Biv) Comparison of LL1 top – RL1 top. (Bv) Comparison of LL2 base. (Bvi) Comparison of LL2 top. (Bvii) Comparison of LL2 base – RL2 base. (Bviii) Comparison of LL1 top – RL1 top. (Bix) Comparison of LL3 base. (Bx) Comparison of LL3 top. (Bxi) Comparison of LL3 base – RL3 base. (Bxii) Comparison of LL3 top – RL3 top. RL1, right side’s latency 1; RL2, right side’s latency 2; RL3, right side’s latency 3; LL1, left side’s latency 1; LL2, left side’s latency 2; LL3, left side’s latency 3; WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. *P<0.05. 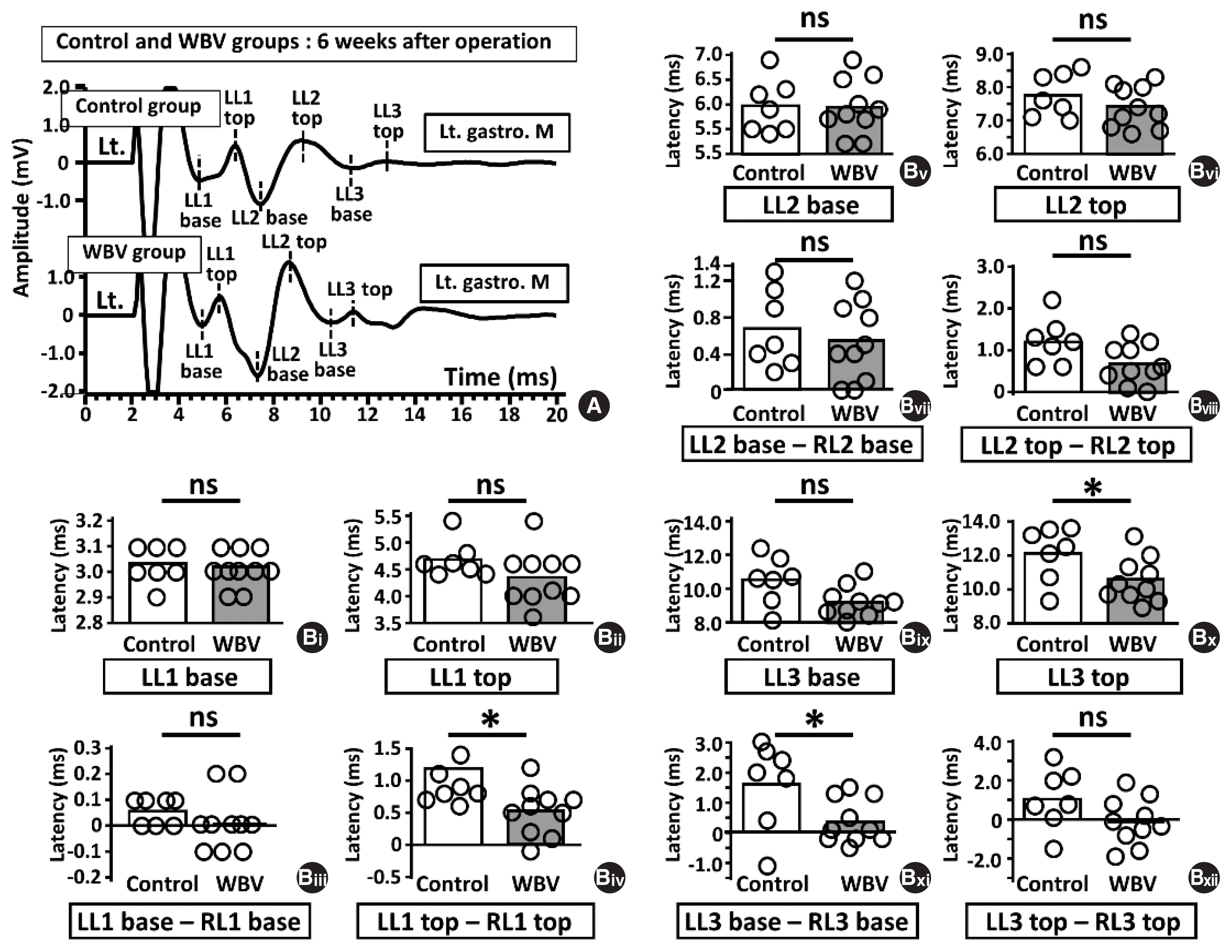
Fig. 7Amplitudes of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) amplitudes at 8 weeks postoperatively. (A) An example of a single bilateral MEPs raw data 8 weeks after sciatic nerve surgery. (Ai) Control group. (Upper panel of Ai) LMS-induced MEPs from Rt. gastro. M. (Lower panel of Ai) LMS-induced MEPs from Lt. gastro. M. (Ab) WBV group. (Upper panel of Aii) LMS-induced MEPs from Rt. gastro. M. (Lower panel of Aii) LMS-induced MEPs from Lt. gastro. M. (B) Bar graphs of amplitude related eight parameters and comparison between the control group (left bar, n=6) and WBV group (right bar, n=8). (Bi) Comparison of LA1. (Bii) Comparison of LA2. (Biii) Comparison of LA3. (Biv) Comparison of LA4. RA1, the amplitude value of (RL1 top–RL1 base); RA2, the amplitude value of (RL1 top–RL2 base); RA3, the amplitude value of (RL2 top–RL2 base); LA4, the amplitude value of (RL2 top–RL3 base); LA1, the amplitude value of (LL1 top–LL1 base); LA2, the amplitude value of (LL1 top–LL2 base); LA3, the amplitude value of (LL2 top–LL2 base); LA4, the amplitude value of (LL2 top–LL3 base); WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. *P<0.05. 
Fig. 8Latencies of lumbar magnetic stimulation (LMS)-induced motor-evoked potentials (MEPs) latencies at 8 weeks postoperatively. (A) An example of a single left MEPs raw data 8 weeks after sciatic nerve surgery. (Ai) Control group. (Aii) WBV group. (B) Bar graphs of latencies related to twelve parameters and comparison between the control group (left bar, n=6) and WBV group (right bar, n=8). (Bi) Comparison of LL1 base. (Bii) Comparison of LL1 top. (Biii) Comparison of LL1 base – RL1 base. (Biv) Comparison of LL1 top – RL1 top. (Bv) Comparison of LL2 base. (Bvi) Comparison of LL2 top. (Bvii) Comparison of LL2 base – RL2 base. (Bviii) Comparison of LL1 top – RL1 top. (Bix) Comparison of LL3 base. (Bx) Comparison of LL3 top. (Bxi) Comparison of LL3 base – RL3 base. (Bxii) Comparison of LL3 top – RL3 top. RL1, right side’s latency 1; RL2, right side’s latency 2; RL3, right side’s latency 3; LL1, left side’s latency 1; LL2, left side’s latency 2; LL3, left side’s latency 3; WBV, whole body vibration; gastro. M, gastrocnemius muscle; ns, not significant. 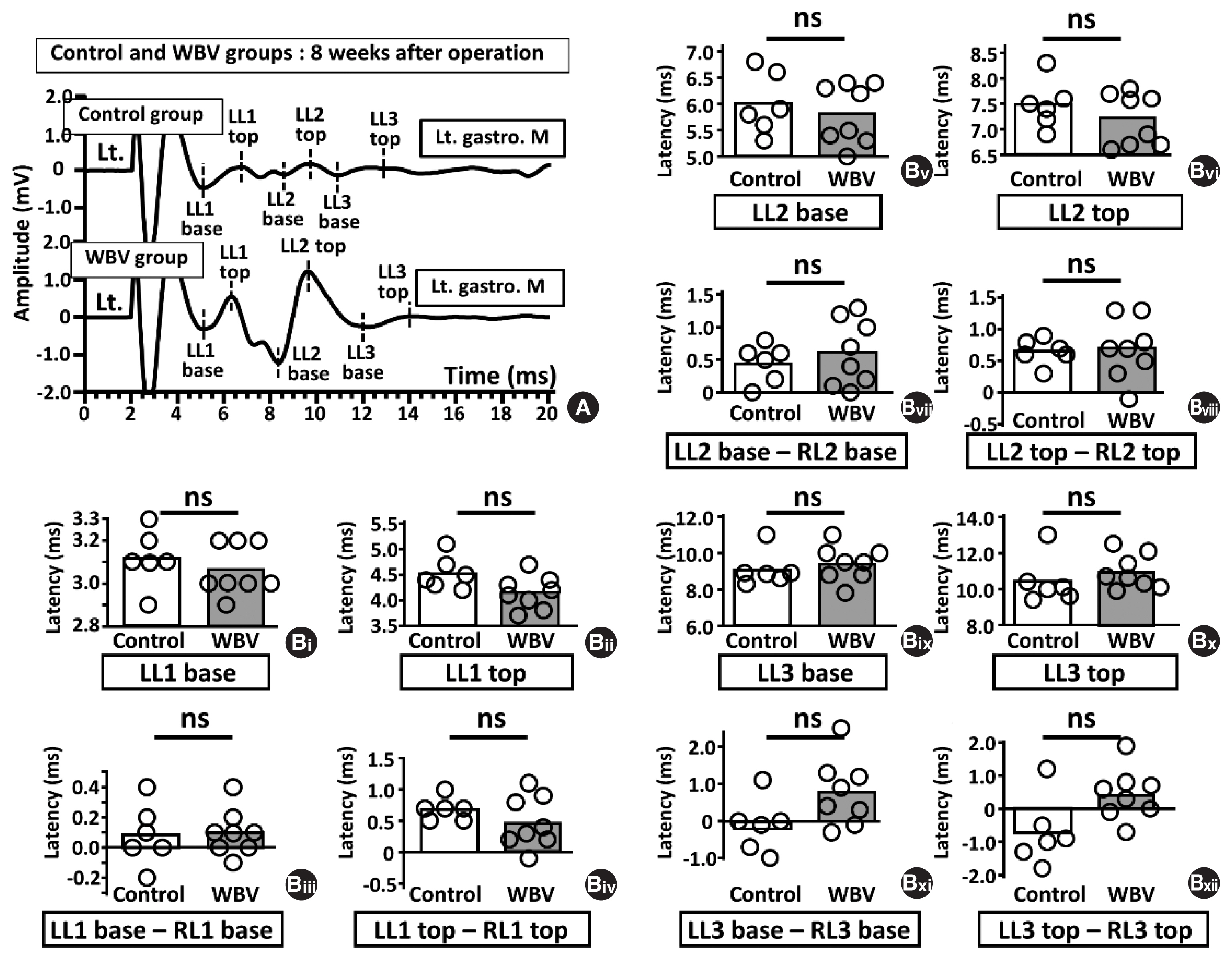
Fig. 9Temporal measurements of bilateral hind-limb dimension. (A) Photos of bilateral hind-limb from the top side. (Ai) A photo of bilateral hind-limb in the control group. (Aii) A photo of bilateral hind-limb at WBV group. (B) Two line graphs of bilateral hind-limb dimension between control and WBV group. (Bi) Two line graphs of the injured left side and a comparison between the control group (white circle) and the WBV group (black circle). (Bii) Two line graphs of the noninjured right side and a comparison between the control group (white circle) and WBV group (black circle). WBV, whole body vibration. *P<0.05. 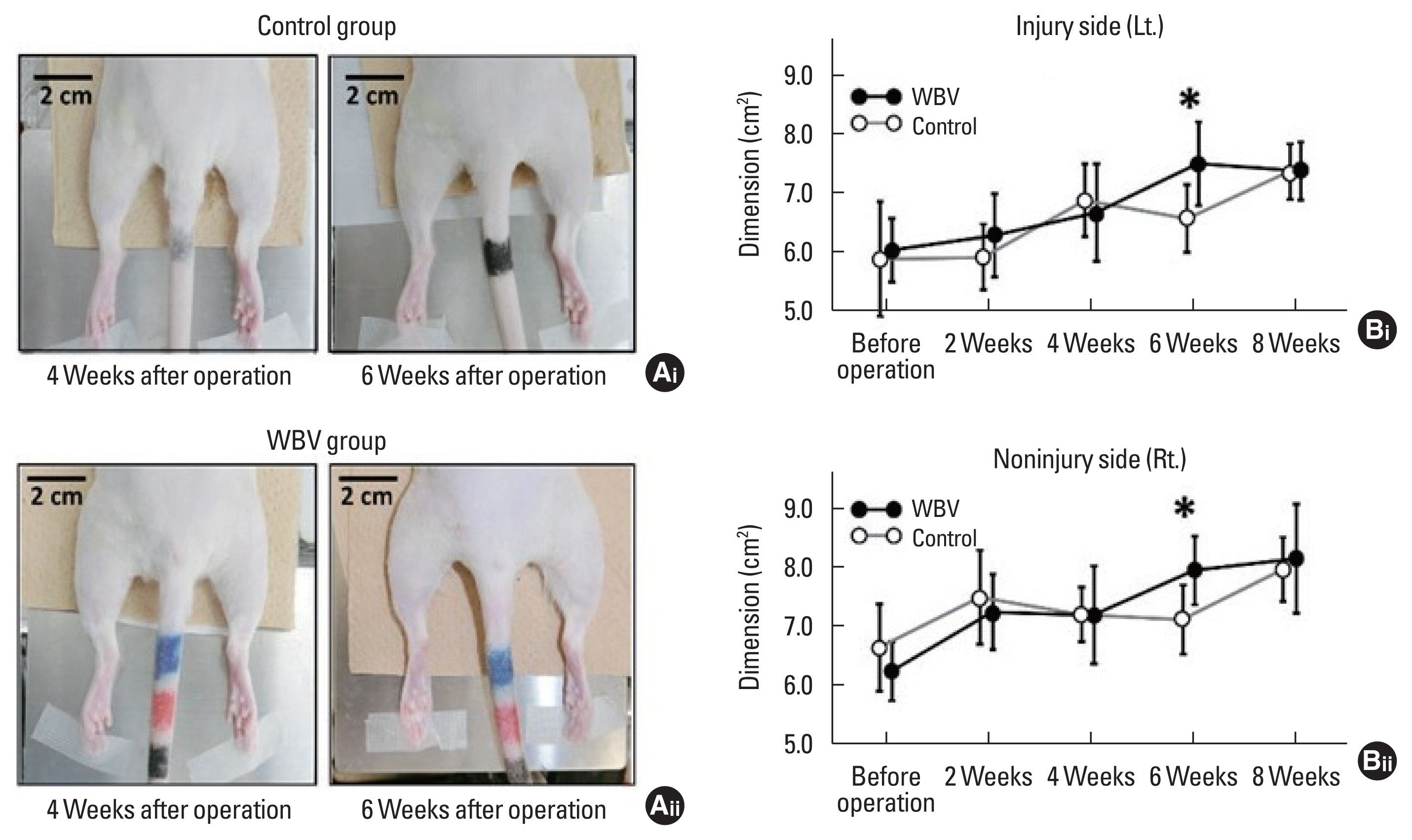
Fig. 10Measurement of both bilateral gastrocnemius dimension and bilateral gastrocnemius weight at 8 weeks postoperatively. (A) Photos of bilateral gastrocnemius from the top side. (Ai) A photo of bilateral gastrocnemius in the control group. (Aii) A photo of bilateral gastrocnemius at WBV group. (B) Bar graphs of both dimension and weight of bilateral gastrocnemius. (Bi) Bar graphs of left gastrocnemius dimension and comparison between the control group (left bar, n=5) and WBV group (right bar, n=6). (Bii) Bar graphs of right gastrocnemius dimension and comparison between the control group (left bar, n=5) and WBV group (right bar, n=6). (Biii) Bar graphs of left gastrocnemius weight and comparison between the control group (left bar, n=5) and WBV group (right bar, n=6). (Biv) Bar graphs of right gastrocnemius weight and comparison between the control group (left bar, n=5) and WBV group (right bar, n=6). WBV, whole body vibration; Lt., left; Rt., right. *P<0.05. 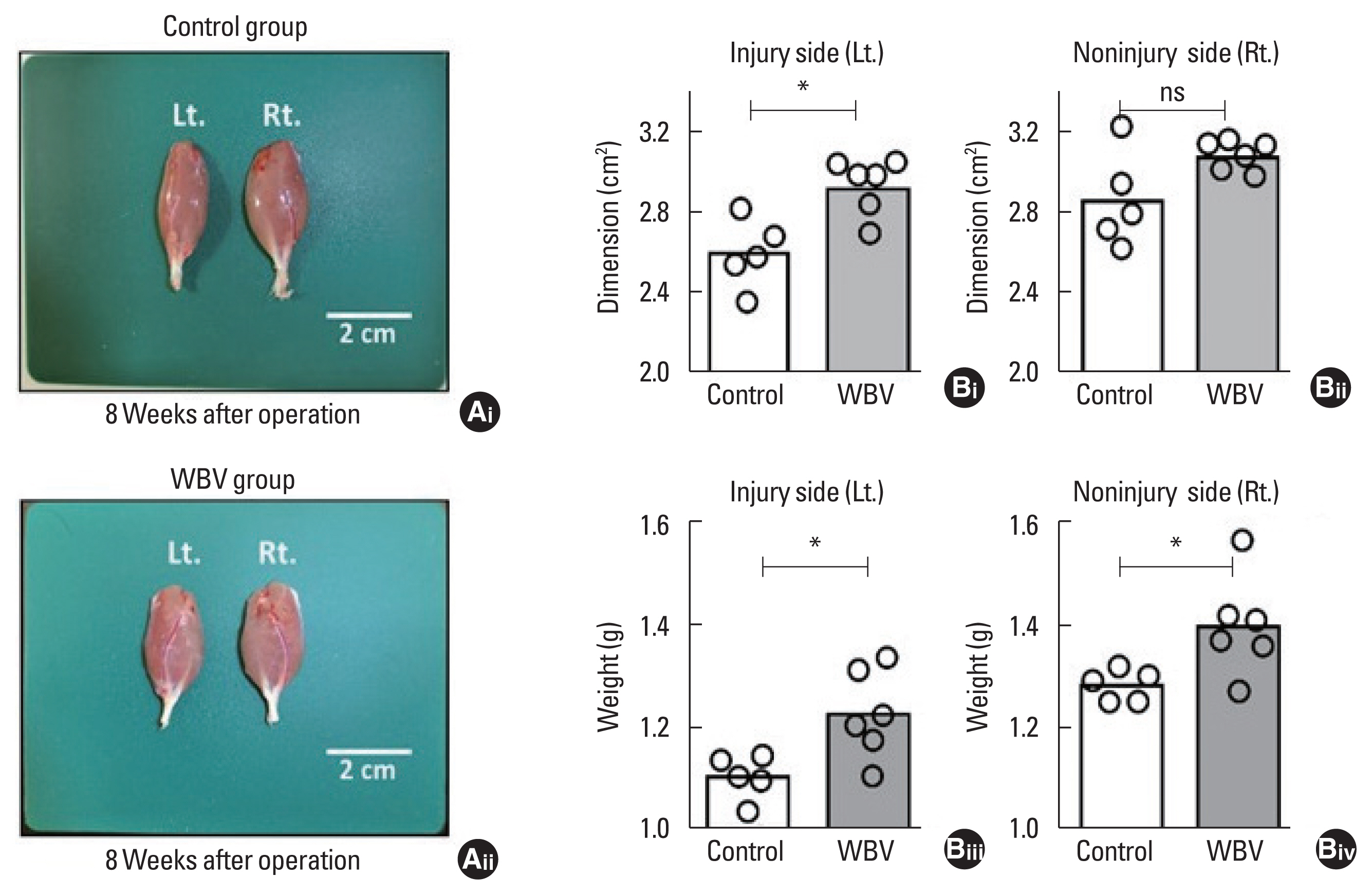
|
|
||||||||||||||||||||||||||||||||||||||||||||||