Treadmill exercise inhibits hippocampal apoptosis through enhancing N-methyl-D-aspartate receptor expression in the MK-801-induced schizophrenic mice
Article information
Abstract
Schizophrenia is a severe mental disorder characterized by abnormal mental functioning and disruptive behaviors. Abnormal expression of N-methyl-D-aspartate (NMDA) receptor, one of the glutamate receptor subtypes, has also been suggested to contribute to the symptoms of schizophrenia. The effect of treadmill exercise on schizophrenia-induced apoptosis in relation with NMDA receptor has not been evaluated. In the present study, we investigated the effect of treadmill exercise on neuronal apoptosis in the hippocampus using MK-801-induced schizophrenic mice. MK-801 was intraperitoneally injected once a day for 2 weeks. The mice in the exercise groups were forced to run on a treadmill exercise for 60 min, once a day for 2 weeks. In the present results, repeated injection of the NMDA receptor antagonist MK-801 reduced expression of NMDA receptor in hippocampal CA2-3 regions. MK-801 injection increased casapse-3 expression and enhanced cytochrome c release in the hippocampus. The ratio of Bax to Bcl-2 was higher in the MK-801-induced schizophrenia mice than the normal mice. In contrast, treadmill exercise enhanced NMDA receptor expression, suppressed caspae-3 activation and cytochrome c release, and inhibited the ratio of Bax to Bcl-2. Based on present finding, we concluded that NMDA receptor hypofunctioning induced neuronal apoptosis in MK-801-induced schizophrenic mice. Treadmill exercise suppressed neuronal apoptosis through enhancing NMDA receptor expression in schizophrenic mice.
INTRODUCTION
Schizophrenia is a severe mental disorder characterized by abnormal mental functioning and disruptive behaviors (Lewis and Lieberman, 2000), occurs in adolescence or early adulthood. This disease has chronic and disruptive effects on cognition (Ross et al., 2006). The symptoms of schizophrenia are divided into three major categories. The first category is positive symptoms, such as thought disorders, hallucinations, and fantasies, which are not openly exhibited by normal people. The second category is negative symptoms characterized by the lack of natural behaviors, such as disability of social interactions, mood disorders, and lack of enjoyment. The third category includes cognitive dysfunction. The exact mechanism of this illness has not been evaluated, however, congenital brain trauma, viruses, neurotoxins, and nutritional deficiency have been suggested as the one of the causes of schizophrenia. One of the representative hypotheses is dopamine dys-function, such as imbalance between dopamine and glutamate or dysfunction in glutamate receptors (Carlsson and Carlsson, 1990; Carlsson et al., 1999). Abnormal expression of N-methyl-D-aspartate (NMDA) receptor, one of the glutamate receptor subtypes, has also been suggested to contribute to the symptoms of schizophrenia (Coyle, 2006).
MK-801 is one of the NMDA receptor antagonists, and this agent has been used for inducing schizophrenia-like symptoms (Yu et al., 2011). NMDA receptor regulates neuronal communication and synaptic function in the central nervous system (Kohr, 2006). Patients with schizophrenia, in particular, exhibit dysfunction in neural networks in various brain regions, such as the frontal cortex, temporal cortex, hippocampus, and subcortical region (Kuppberg and Heckers, 2000). Enhancement of apoptosis reduces the survival rate of neurons and neuroglia in various stages of neural development, causing synaptic deficits in the schizophrenia (Glantz et al., 2006). The relationship of apoptosis to the schizophrenia is already suggested (Benes et al., 2003; Jarskog et al., 2004).
During the process of apoptosis in the brain, cytochrome c is released from the mitochondrial cytoplasm, under the regulation of Bax (a pro-apoptotic protein) and Bcl-2 (an anti-apoptotic factor). When Bax is activated, release of cytochrome c is increased, and this activates caspases, leading to apoptosis through various proteins, such as caspase-1, caspase-3, and caspase-4. In this process, Bcl-2 directly suppresses apoptosis and hence inhibits the downstream activation of caspase-9 and caspase-3. A high ratio of Bax to Bcl-2 accelerates apoptosis, while a low ratio of Bax to Bcl-2 suppresses apoptosis (Hwang et al., 2013). Abnormally accelerated apoptosis triggers a variety of brain diseases (Jarskog et al., 2004; Kim et al., 2013a; Yoon et al., 2013).
Exercise is known as the non-pharmaceutical treatment for the many brain disorders. Exercise attenuates apoptotic neuronal cell death in the neuropsychiatric diseases (Cho et al., 2013; Kim et al., 2013b; Yoon et al., 2013). Exercise can have healthful effects on both physical and mental health in schizophrenia patients (Scheewe et al., 2013). Anti-apoptotic effect of exercise on neuronal cells has been well documented, however, the effect of treadmill exercise on schizophrenia-induced apoptosis in relation with NMDA receptor expression has not been evaluated. In the present study, we investigated the effect of treadmill exercise on neuronal apoptosis in the hippocampus using MK-801-induced schizophrenic mice.
MATERIALS AND METHODS
Animal and experimental design
Male C57BL/6 mice (6 weeks old weighing 25±2 g) were used in this study, The mice were individually housed in plastic cages at a controlled temperature (23±2°C) and maintained under lighting (08:00 to 20:00 h) conditions with food and water available ad libitum. Experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The mice were divided into four groups (n=10 in each group): the control group, the control and exercise group, the MK-801 injection group, and the MK-801 injection and exercise group.
Preparation of MK-801-induced schizophrenia model in mice
MK-801 (dizocilpine maleate) is a non-competitive NMDA receptor antagonist, and it was purchased from the Sigma Chemical Co. (St. Louis, MO, USA). MK-801 was prepared as a stock solution (1 mg/mL, dissolved in saline) and 0.6 mg/kg of it was intraperitoneally injected once a day for 2 weeks, as the previously described method (Yu et al., 2011).
Exercise protocol
The mice in the exercise group were forced to run on a motorized treadmill for 30 min once a day for 2 weeks. The exercise load consisted of running at a speed of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and 8 meters/min for the last 50 min, with a 0° inclination. The mice in the non-exercise groups were left on the treadmill without running for the same period as the exercise groups.
Tissue preparation
The mice were sacrificed 15 days after beginning the experiment. At the beginning of the sacrificial procedure, the animals were weighed and overdosed with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the mice were transcardially perfused with 50 mM of phosphate-buffered saline (PBS) and then with 4% of paraformaldehyde in a 100 mM phosphate buffer (PB) at a pH 7.4. The brains were dissected, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Brains were rapidly frozen in a deep freezer at −80°C and serial coronal sections of 40 µm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry for NMDA receptor and caspase-3
To visualize NMDA receptor and caspase-3, immunohistochemistry for NMDA receptor in the CA2-3 and dentate gyrus hippocampus were performed, according to the previously described method (Hwang et al., 2013). The sections were incubated in PBS for 10 min, and then washed three times in the same buffer. The sections were then incubated in 1% H2O2 for 30 min. The sections were selected from each brain and incubated overnight with rabbit anti-NMDA receptor 1 and anti-caspase-3 antibody (1:500, abcam, Cambridge, UK; 1:800, cell signaling), and then with biotinylated rabbit secondary antibody (1:200; Vector Laboratories) for another 1 h. The secondary antibody was amplified with the Vector Elite ABC kit® (1:100; Vector Laboratories). Antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% 3,3´ diaminobenzidine (DAB), and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Per-mount®.
Western blot for Bax, Bcl-2, and cytochrome c
Western blot was performed according to the previously described method (Hwang et al., 2013). The hippocampus were collected, then immediately frozen at −70°C. Protein from each hippocampus and dorsal raphe nuclei were extracted. The tissues were homogenized with lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM Mg-Cl2·6H2O, 1 mM EGTA, 1 mM PMSF, 1 mM Na2VO4, and 100 mM NaF, then ultra- centrifuged at 50,000 rpm for 1 h. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein (30 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Anti-Bax (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Bcl-2 (1:1,000; Santa Cruz Biotechnology), anti-cytochrome c (1:1,000; Santa Cruz Biotechnology), and anti-β-actin (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for β-actin, Bax, Bcl-2, and cytochrome c were used as the secondary antibodies. Experiments were performed in normal laboratory conditions and at room temperature, except for the transferred membranes. Transferred membranes were performed at 4°C with the cold pack and pre-chilled buffer. Band detection was performed using the enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology).
Data analysis
For confirming the expressions of Bax, Bcl-2 and cytochrome c, the detected bands were calculated densitometrically using Molecular AnalystTM, version 1.4.1 (Bio-Rad). The number of NMDA receptor in the hippocampal CA2-3 and the number caspase-3-positive cells in the hippocampal dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and the areas of hippocampal CA2-3 and hippocampal dentate gyrus were measured by Image-Pro® Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). These numbers were expressed as the numbers of cells/mm2. The data were analyzed with one-way ANOVA and then Duncan post-hoc tests. All values are expressed as the mean±standard error of the mean (SEM), and P value<0.05 was considered significant.
RESULTS
Effect of treadmill exercise on the number of NMDA receptor in the hippocampal CA2-3
The effect of treadmill exercise on the number of NMDA receptor in the hippocampal CA2-3 is presented in Fig. 1. The number of NMDA receptors-positive cells was 992.19±70.38/mm2 in the control group, 1,146.54±96.68/mm2 in the control and exercise group, 516.10±32.57/mm2 in the MK-801-injection group, and 793.78±27.29/mm2 in MK-801-injection and exercise group. The number of NMDA receptor-positive cells in the CA2-3 was decreased by MK-801 injection, in contrast, treadmill exercise increased this number in the MK-801-induced schizophrenic mice.
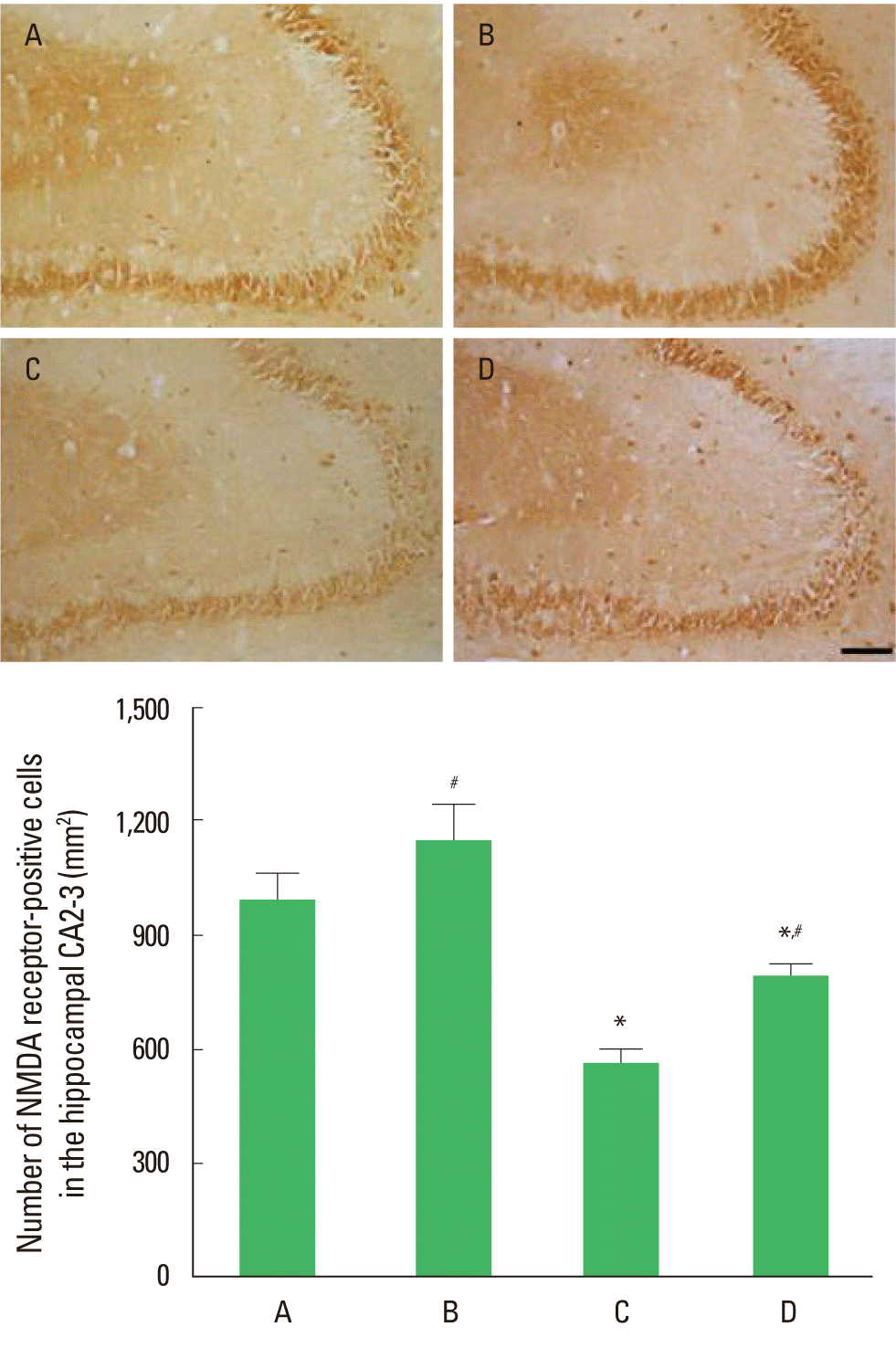
Effect of the treadmill exercise on NMDA receptor in hippocampal CA2-3 of schizophrenic mice. Upper: Photomicrographs of NMDA receptor-positive cells. The scale bar represents 100 µm. Lower: Number of NMDA receptor-positive cells in each group. (A) Control group, (B) control and exercise group, (C) MK-801-injection group, (D) MK-801-injection and exercise group. The data are presented as the mean± standard error of the mean (SEM). *Represents P< 0.05 compared to the control group. #Represents P< 0.05 compared to the MK-801-injection group.
Effect of treadmill exercise on the number of caspase-3-positive cells in hippocampal dentate gyrus
The effect of treadmill exercise on the number of capase-3-positive cells in the hippocampal dentate gyrus is presented in Fig. 2. The number of caspase-3-positive cells in the dentate gyrus was 154.48±23.48/mm2 in the control group, 141.02±4.56/mm2 in the control and exercise group, 229.05±8.97/mm2 in the MK-801-injection group, and 188.43±14.54/mm2 in MK-801-injection and exercise group. Expression of caspase-3 in the dentate gyrus was decreased by MK-801 injection, in contrast, treadmill exercise increased caspase-3 expression in the MK-801-induced schizophrenic mice.
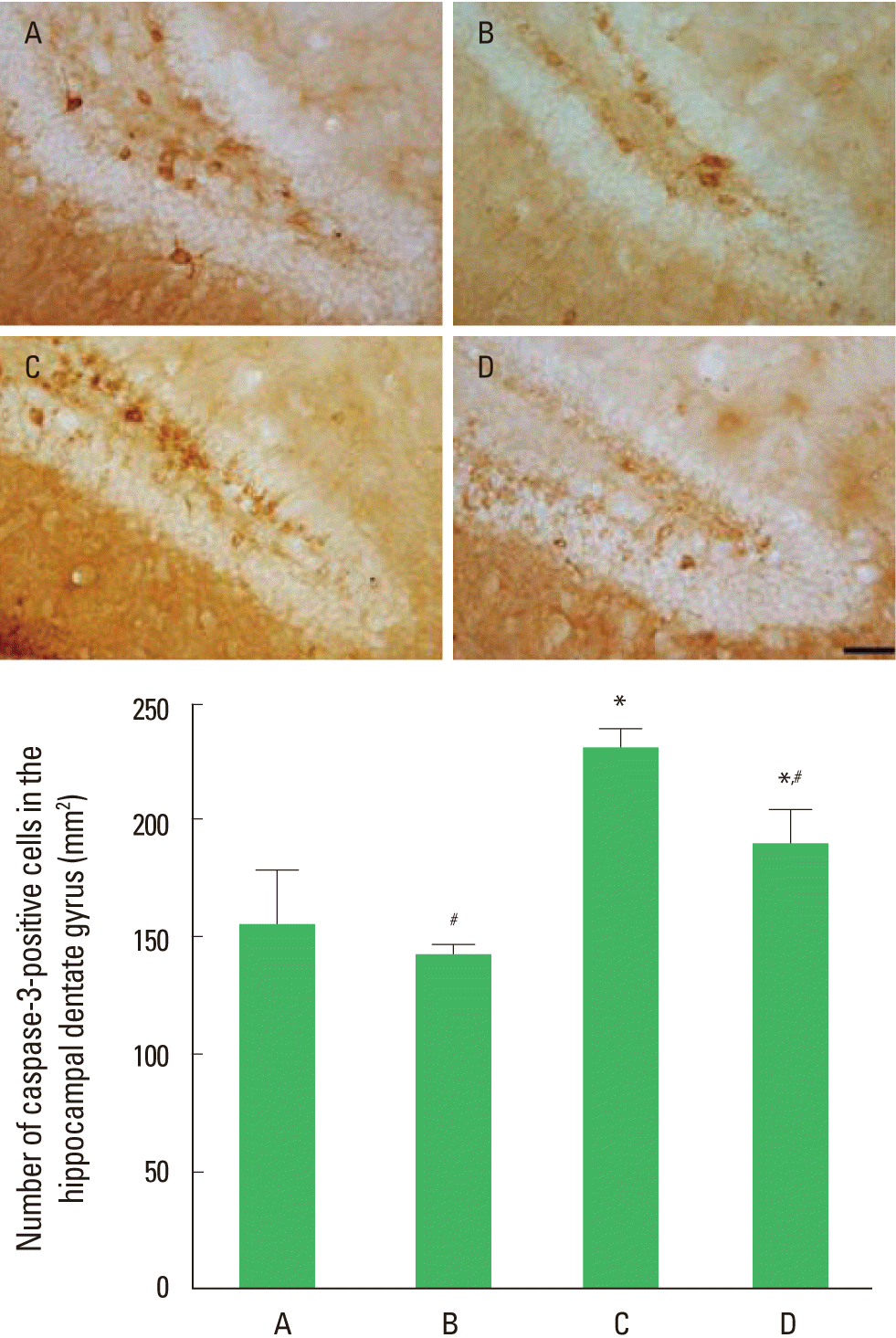
Effect of the treadmill exercise on caspase-3 expression in hippocampal dentate gyrus of schizophrenic mice. Upper: Photomicrographs of caspase-3-positive cells. The scale bar represents 50 µm. Lower: Number of caspase-3-positive cells in each group. (A) Control group, (B) control and exercise group, (C) MK-801-injection group, (D) MK-801-injection and exercise group. The data are presented as the mean± standard error of the mean (SEM). *Represents P< 0.05 compared to the control group. #Represents P< 0.05 compared to the MK-801-injection group.
Effect of treadmill exercise on Bax and Bcl-2 expressions in the hippocampus
The effect of treadmill exercise on the expressions of Bax and Bcl-2 in the hippocampus is presented in Fig. 3. The level of Bax in the control group was set to 1.00. The level of Bax was 0.92± 0.03 in the control and exercise group, 1.43±0.06 in the MK-801-injection group, and 1.14±0.04 in the MK-801-injection and exercise group. The expression of Bax was increased by MK-801 injection, in contrast, treadmill exercise decreased Bax expression in the MK-801-induced schizophrenic mice.

Effect of the treadmill exercise on Bax and Bcl-2 expressions in hippo-campus of schizophrenic mice. Upper: Effect of the treadmill exercise on the expression of Bax. Middle: Effect of the treadmill exercise on the expression of Bcl-2. Lower: Effect of the treadmill exercise on the ratio of Bax to Bcl-2. (A) Control group, (B) control and exercise group, (C) MK-801-injection group, (D) MK-801-injection and exercise group. The data are presented as the mean ± standard error of the mean (SEM). *Represents P< 0.05 compared to the control group. #Represents P< 0.05 compared to the MK-801-injection group.
The level of Bcl-2 in the control group was set to 1.00. The level of Bcl-2 was 1.22±0.01 in the control and exercise group, 0.74 ±0.01 in MK-801-injection group, and 0.89±0.03 in MK-801-injection and exercise group. The expression of Bcl-2 was decreased by MK-801 injection, in contrast, treadmill exercise decreased Bcl-2 expression in the MK-801-induced schizophrenic mice.
The ratio of Bax to Bcl-2 was calculated. The ratio of Bax to Bcl-2 in the control group was set to 1.00. The ratio of Bax to Bcl-2 was 0.75±0.02 in the control and exercise group, 1.92±0.08 in the MK-801-injection group, and 1.28±0.04 in the MK-801-injection and exercise group. The ratio of Bax to Bcl-2 was increased by MK-801 injection, in contrast, treadmill exercise decreased this ratio in the MK-801-induced schizophrenic mice.
Effect of treadmill exercise on cytochrome c expression in the hippocampus
The effect of treadmill exercise on cytochrome c expression in the hippocampus is presented in Fig. 4. The level of cytochrome c expression in the control group was set to 1.00. The level of cytochrome c expression was 1.09±0.03 in the control and exercise group, 2.02±0.09 in the MK-801-injection group, and 1.67± 0.08 in the MK-801-injection group that exercised group. The expression of cytochrome c was increased by MK-801 injection, in contrast, treadmill exercise decreased cytochrome c expression in the MK-801-induced schizophrenic mice.

Effect of the treadmill exercise on cytochrome c expression in hippocampus of schizophrenic mice. (A) Control group, (B) control and exercise group, (C) MK-801-injection group, (D) MK-801-injection and exercise group. The data are presented as the mean± standard error of the mean (SEM). *Represents P< 0.05 compared to the control group. #Represents P< 0.05 compared to the MK-801-injection group.
DISCUSSION
Schizophrenia is complex neuropsychiatric disorders and dys-function of neural networks is related to the apoptosis (Benes et al., 2003; Jarskog et al., 2004). In the present study, repeated injection of the NMDA receptor antagonist MK-801 reduced expression of NMDA receptor in hippocampal CA2-3 regions, indicating that MK-801 induced schizophrenia-like biochemical alterations in the brain.
NMDA receptor hypofunction initiates apoptotic cell death through activation of caspase-3 (Takadera et al., 1999). NMDA receptor blockage induces apoptosis by activating proteolytic enzymes, such as caspase-3, in the primary cultured hippocampal neurons (Hardingham et al., 2002). Conversely, activation of synaptic NMDA receptor has a protective effect on neurons (Hardingham et al., 2002). Brain of schizophrenia exhibits neuronal loss through cell death that is the visible symptom in the hippocampal region of schizophrenia (Csernansky et al., 2006). In the present study, MK-801 injection increased casapse-3 expression and enhanced cytochrome c release in the hippocampus, indicating that NMDA hypofunction induced neuronal apoptosis.
The neuronal injury of schizophrenia is the abnormally enhanced apoptosis (Glantz et al., 2006). Bcl-2 was reduced in patients with schizophrenia (Jarskog et al., 2000), and the ratio of Bax to Bcl-2 was 50% higher in schizophrenia patients compared to the non-psychiatric comparison subjects (Jarskog et al., 2004). In the present study, the ratio of Bax to Bcl-2 was higher in the MK-801-induced schizophrenia mice than the normal mice, indicating that inducing of schizophrenia enhanced neuronal apoptosis.
Exercise also exerts improving effect on physical and mental health in schizophrenia patients (Acil et al., 2008; Scheewe et al., 2013). Exercise play as the essential factor for NMDA receptor activity and NMDA receptor release in the hippocampus (Dietrich et al., 2005; Kitamura et al., 2003). Exercise not only increases the expression of phosphorylated NMDA receptor but also enhances the activity of NMDA receptor (Hardingham and Bading, 2010). In the present study, treadmill exercise enhanced NMDA receptor expression in the schizophrenia mice, indicating that tread-mill exercise restored NMDA receptor.
Exercise increased neuron density of hippocampus without altering apoptosis in adolescent rats (Uysal et al., 2005). Treadmill exercise suppressed Bax expression, inhibited casapse-3 activation, and increased Bcl-2 expression in traumatic brain injury (TBI) (Kim et al., 2010). Following exercise training, hippocampal volume was increased in the schizophrenia patient (Pajonk et al., 2010). In the present study, treadmill exercise suppressed caspae-3 activation and cytochrome c release, and inhibited the ratio of Bax to Bcl-2, indicating that treadmill exercise exerted anti-apoptotic effect.
Based on present finding, we concluded that NMDA receptor hypofunctioning induced neuronal apoptosis in MK-801-induced schizophrenic mice. Treadmill exercise suppressed neuronal apoptosis through enhancing NMDA receptor expression in schizophrenic mice. The present study suggests that treadmill exercise may provide a potential therapeutic strategy for the schizophrenia patients.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012 S1A5B5A07037274).