Association of BID SNPs (rs8190315 and rs2072392) and clinical features of benign prostate hyperplasia in Korean population
Article information
Abstract
Exercise has beneficial effect on cancer apoptosis and benign prostatic hyperplasia (BPH). The BH3 interacting domain death agonist (BID) gene expression is associated with apoptosis or cell proliferation. In this study, we investigated the association between BID single nucleotide polymorphisms (SNPs) and the development, prostate volume, and international prostate symptom score (IPSS) of BPH. In 222 BPH males and 214 controls, two SNPs in BID [rs8190315 (Ser56Gly), and rs2072392 (Asp106Asp)] were genotyped and analyzed using multiple logistic regression models. In the result, the genotype and allele frequencies of rs8190315 and rs2072392 were not associated with BPH development or IPSS, however, the allele frequencies [odd ratio (OR)= 1.90, 95% confidence interval (CI)= 1.07–3.41, P= 0.03] and genotype frequencies (in dominant model, OR= 1.94, 95% CI= 1.01–3.74, P= 0.42) of rs8190315, and the genotype frequencies of rs2072392 (in dominant model, OR= 1.94, 95% CI= 1.01–3.74, P= 0.42) were associated with increased prostate volume. We propose that rs8190315 and rs2072392 of BID may contribute to the disease severity of BPH.
INTRODUCTION
Exercise has beneficial effects on human body organs (Kim, 2014; Kim et al., 2004). Lifestyle factors, including body mass index (BMI) and physical activity (Lee et al., 2014; Marshall et al., 2014), are associated with lower urinary tract symptoms (LUTS) or benign prostate hyperplasia (BPH) of aged men, and it has been reported that increased physical activities tend to exert a protective role on prostate against the development of BPH (Barnard et al., 2008; Lee et al., 2014; Sea et al., 2009). Moreover, There are evidences suggest that increased physical activities may elicit apoptosis in cancer cells (Bartlett et al., 2014; Ergun et al., 2013; Hojman et al., 2011; Zheng et al., 2008), and such effects have also been shown in prostate cells (Barnard et al., 2008; Meyer et al., 2013; Teixeira et al., 2012; Zheng et al., 2012).
Cellular pathways of apoptosis may be affected by the BH3 interacting domain death agonist (BID) gene, because which is involved in the control of apoptosis by activation of caspase-8. Caspase-8 has been intensively studied in the cancer cells (Liu et al., 2014; Sharifi et al., 2014), as it is related to the cell cycle and apoptosis (Bissoyi et al., 2014; Wei et al., 2013) which are directly related to modulation of cellular proliferative diseases. Caspase-8 pathway includes BAX, a death agonist, and BCL2, a death antagonist. And BID may heterodimerize with BAX or BCL2, to activate apoptosis (Wang et al., 1996).
The BID gene polymorphisms may affect the development of immunoglobulin A nephropathy (Park et al., 2014), and ossification of the posterior longitudinal ligament (Chon et al., 2014). Moreover, BID expression is related to cellular reaction to cholesterol oxidation (Jung et al., 2014), insulin resistance (Alkhouri et al., 2010), effect of FTY720 to chronic myelogenous leukemia cells (Kiyota et al., 2013), and interestingly, polymorphism of BID may be related to breast cancer (Gaudet et al., 2009).
Among them, cholesterol metabolism and glucose intolerance have been known to be closely associated with BPH (Vignozzi et al., 2013), and the relation to breast cancer suggests that BID may modulate the sex hormone affected cell proliferation (Gaudet et al., 2009). However, there was no study reported whether there is association of BID polymorphism and BPH development in human. Therefore, in this study, we investigated the relationship between BID single nucleotide polymorphisms (SNPs) and BPH in a Korean population.
MATERIALS AND METHODS
Study subjects
Two-hundred and 22 BPH patients (mean age 65.5+10.3 yr) (Table 1) were recruited from Kyung Hee Medical Center. The lower urinary tract symptoms (LUTS) of BPH patients were scaled using the international prostate symptom score (IPSS). Prostate sizes of the patients were measured using transrectal ultrasound. Any patients with urinary tract infections, prostate cancer, a neurogenic bladder, uncontrolled diabetes mellitus, and/or cardiovascular diseases were not included in this study. In the analysis of clinical features, BPH patients were divided into groups based on the following features: prostate volume (cutoff at 30 mL) and IPSS (cutoff at 20). The controls were 214 aged male (mean age 61.9±8.2 yr) without any of severe diseases mentioned above. The Ethics Review Committee of Medical Research Institute, Kyung Hee University School of Medicine, Seoul, Korea has admitted the Ethical approval of this study.
SNP selection and genotyping
We selected 2 SNPs in BID: rs8190315 (Ser56Gly), and rs 2072392 (Asp106Asp). They were searched from the SNP database in NCBI (http://www.ncbi.nlm.nih.gov/SNP), and reviewed to select the region in exons and near exons. Additionally, the two SNPs were previously studied polymorphism of BID in the Korean population (Chon et al., 2014; Park et al., 2014). Peripheral blood of all patients were sampled in ethylenediaminetetraacetic acid blood tube, stored in −20°C freezer before the extraction of genomic DNA. Genomic DNA was extracted with QIAamp® DNA extraction kit (QIAGEN, Valencia, CA, USA). In the amplification of DNA, Polymerase chain reactions (PCRs) using the following primers for each SNPs was performed: rs8190315 (sense, 5′-ACAGGGTCCCTCAGCTCTCTGG-3′; antisense, 5′-GCCTA CCTGCCTCTATTCTTCC-3′, 340 bp) and rs2072392 (sense, 5′-GGATGAGTGCATCACAAACCTA-3′; antisense, 5′-AGCACTGGAGGGAATCAAGTAG-3′, 367 bp). The primers used in this study were in a previous study BID 24621205. Direct sequencing was done with ABI PRISM 3730XL analyzer (PE Applied Biosystems, Foster City, CA, USA) and analyzed with SeqManII software (DNASTAR, Madison, WI, USA).
Statistical analysis
In the analysis of genotypes and allele frequencies, SNPStats (http://bioinfo.iconcologia.net/index.php?module=Snpstats) and SPSS 20.0 (SPSS Inc., Chicago, IL, USA) were used to obtain odds ratios (ORs), 95% confidence intervals (CIs), and P-values of multiple logistic regression models. Each component of the model was: codominant1 (major allele homozygotes vs heterozygotes), codominant2 (major allele homozygotes vs minor allele homozygotes), dominant (major allele homozygotes vs heterozygotes+minor allele homozygotes), recessive (major allele homozygotes+heterozygotes vs minor allele homozygotes), and log-additive (major allele homozygotes vs heterozygotes vs minor allele homozygotes) (Kim et al., 2013). Hardy-Weinberg equilibrium of each SNP was estimated by SNPstats. Age was adjusted as a covariate in statistical analysis. The linkage disequilibrium (LD) block and haplotypes between pairs of SNPs were tested with Haploview 4.2 software (Daly Lab, Cambridge, MA, USA). Significant level of P-value was regarded at <0.05.
RESULTS
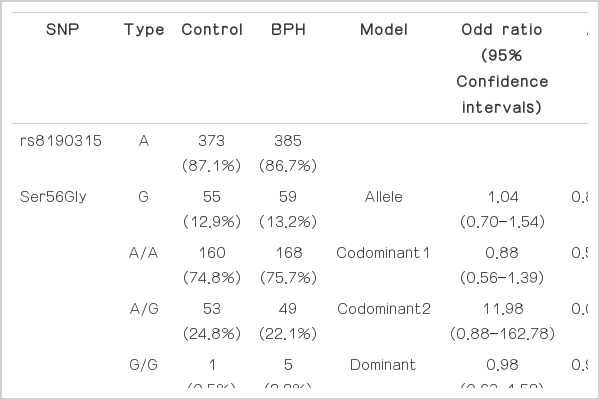
The frequencies of genotype and allele of two BID SNPs in control and patients with BPH were displayed in the Table 2, however, there was no significant association between the SNPs and BPH development. The Hardy-Weinberg equilibrium P-value of the SNPs were >0.05 (data not shown).
The genotypes and allele distributions of BID SNPs in the groups divided based on prostate volume are shown in Table 3. The allele frequencies of rs8190315 were associated with increased volume of prostate in BPH patients {odd ratio (OR)=1.90, 95% confidence interval (CI)=1.07–3.41, P=0.03}, showing about 1.8-fold increase of minor allele in the BPH group (16.5%) compared to controls (9.4%). The genotypes of rs8190315 were also associated with increased prostate volume in dominant model (OR=1.94, 95% CI=1.01–3.74, P=0.42). And the genotype of rs2072392 were associated with increased prostate volume in dominant model (OR=1.94, 95% CI=1.01–3.74, P=0.42), however, the allele frequencies of rs2072392 were not associated with prostate volume in BPH patients.
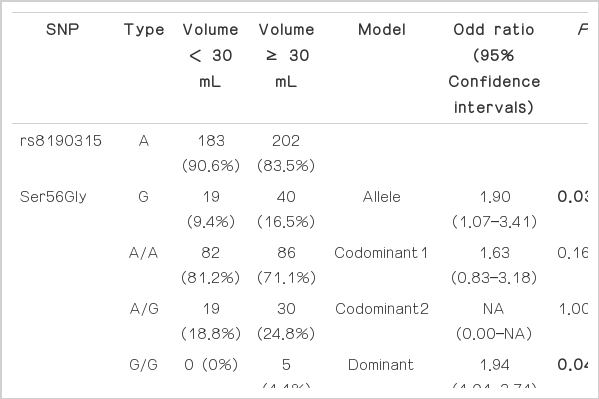
The genotypes and allele distributions of BID SNPs in the groups divided based on prostate volume (30 mL)
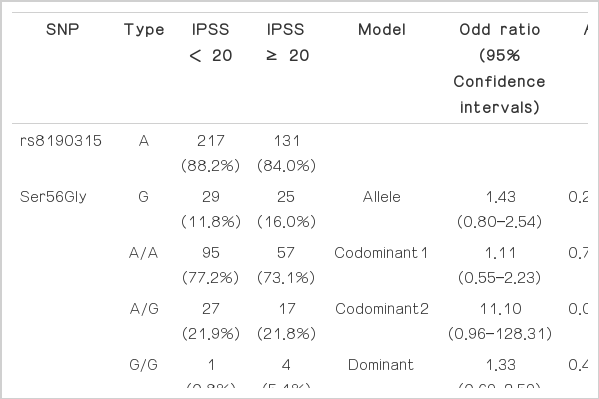
The genotypes and allele distributions of BID SNPs in the groups divided based on IPSS are shown in Table 4, however, there was no significant association between the SNPs and IPSS of the BPH patients.
DISCUSSION
In our study result, BID SNPs were not associated with development of BPH, however, prostate volume of BPH patients were associated with BID SNPs. There were three previous studies regarding the SNPs investigated in this study in Pubmed. rs8190315 was associated with the risk of breast cancer in European woman (Gaudet et al., 2009), meanwhile, rs8190315 and rs2072392 were associated with the proteinuria levels of IgAN patients (Park et al., 2014), and risk of developing OPLL in Korean population (Chon et al., 2014). MAF of rs8190315 were 0.018 in European, 0.062 in Sub-Saharan African, 0.128 in Han Chinese, and 0.106 in Japanese; MAF of rs2072392 were 0.008 in European, 0.075 in Sub-Saharan African, 0.122 in Han Chinese, and 0.125 in Japanese. There are more than five-fold to ten-fold differences between MAFs of rs8190315 and rs2072392 in European and Asian populations, and it may contributed to the diversion of results. Results of this study accord to previous studies performed in Korean population, that the both of rs8190315 and rs2072392 may affect the volume of enlarged prostate in BPH patients.
Previous reports suggest that, hyperplasia of the prostate may be affected by modulation of stress by cholesterol oxidation (Vignozzi et al., 2013), because oxidized low-density lipoprotein (LDL) may cause decreased apoptosis in progressing BPH lesions (Gonzalez-Chavarria et al., 2014; Lin et al., 2014), however, study result of Pace et al. (Pace et al., 2010) is on the contrary that oxidized LDL was not correlated with BPH. Additionally, regarding the role of BID, variation in BID may have contributed to alteration in apoptosis pathways (Asmarinah et al., 2014). Although there was no reported direct relationship between BPH and BID, however, BPH and prostate cancer have similarity that testosterone drives cellular hyperplasia in both diseases (Negri et al., 2005), and activation of BID was associated with apoptosis of androgen-stimulated prostatic cells (Deeb et al., 2003; Goldberg et al., 2013; Sridhar et al., 2001; Zhu et al., 2007). Our study results may partially accord with those previous studies.
In summary, we suggest that rs8190315 and rs2072392 of BID may contribute to the disease severity of BPH. Regarding the role of BID in modulating apoptotic signal in metabolic syndrome or dyslipidemia, this study result may intensify the role of physical activity in preventing BPH. Our study has some limitations, such as only small population of Korean was analyzed. Therefore, more subjects will be needed to confirm our results in further study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009558)” Rural Development Administration, Republic of Korea.