Role of creatine supplementation in exercise-induced muscle damage: A mini review
Article information
Abstract
Muscle damage is induced by both high-intensity resistance and endurance exercise. Creatine is a widely used dietary supplement to improve exercise performance by reducing exercise-induced muscle damage. Many researchers have suggested that taking creatine reduces muscle damage by decreasing the inflammatory response and oxidative stress, regulating calcium homeostasis, and activating satellite cells. However, the underlying mechanisms of creatine and muscle damage have not been clarified. Therefore, this review discusses the regulatory effects of creatine on muscle damage by compiling the information collected from basic science and sports science research.
INTRODUCTION
Exercise-induced muscle damage occurs after high-intensity resistance and endurance exercise (Santos et al., 2004; Veggi et al., 2013). Exercise-induced muscle damage is classified into primary and the secondary damage (Howatson and van Someren, 2008). Primary muscle damage is related to morphological changes, including sarcomere (Z-disc, I, and A band), sarcolemma, sarcoplasmic reticulum, and cytoskeletal elements (Clarkson and Hubal, 2002). Secondary muscle damage occurs due to impaired calcium homeostasis and the inflammatory response (Beaton et al., 2002; Tidball, 2005). Impaired calcium homeostasis due to sarcoplasmic reticulum dysfunction is activated by calpain-3, which is a calcium-activated neutral protease (Beaton et al., 2002), that increases muscle damage and protein degradation (Murphy, 2010). Additionally, inflammatory response may promote further muscle damage. Neutrophils and macrophages invade the damaged site, facilitate phagocytosis, and secrete substances that induce oxidative stress (Tidball, 2005). This phenomenon may lead to a decrease in maximal strength and increase delayed-onset muscle soreness and muscle proteins, such as creatine kinase (CK) and lactate dehydrogenase (LDH) in the blood (Clarkson and Hubal, 2002).
Use of dietary supplements is a recommended scheme to attenuate exercise-induced muscle damage (Sousa et al., 2014). Creatine has been used as a dietary supplement for a long time by many athletes and others (Bird, 2003). Creatine (N-aminoiminomethyl-N-methylglycine) is a naturally generated endogenous guanidine compound synthesized in the kidneys, pancreas, and liver from methionine, glycine, and arginine (Bemben and Lamont, 2005) and released into the blood (D’Antona et al., 2014). Most creatine is localized in skeletal muscle and stored as creatine phosphate (PCr). CK and PCr play a pivotal role in short-term (only a few seconds) exercise (Bird, 2003). High creatine levels are found naturally in meat and fish (D’Antona et al., 2014) and intramuscular PCr levels can be increased by approximately 20% by using a creatine supplement (Harris et al., 1992).
The ergogenic effect of creatine is well-known to improve exercise performance such as explosive muscle power (Claudino et al., 2014; Zuniga et al., 2012) and increased lean body mass after resistance exercise (Candow et al., 2014; Chilibeck et al., 2004). Several studies have reported that creatine promotes recovery by attenuating muscle damage after eccentric exercise (Cooke et al., 2009; Rosene et al., 2009). Cooke et al. (2009) indicated that creatine supplementation may help rescue maximal strength and inhibit CK release due to high intensity exercise. In addition, Rosene et al. (2009) concluded that creatine improves maximal strength after exercise. In contrast, other studies have reported that creatine does not reduce muscle damage after eccentric exercise (Mckinnon et al., 2012; Rawson et al., 2001).
Despite that creatine potentially reduces muscle damage, it has not generally been used in the sports rehabilitation field. The aim of this review was to introduce the effects of taking creatine on exercise-induced muscle damage.
CREATINE AND EXERCISE-INDUCED MUSCLE DAMAGE
An initial study by Warren et al. (2000) verified the effect of creatine on exercise-induced muscle damage. In this study mice performed 150 eccentric muscle contractions in response to electric stimulation after ingesting 0.5 or 1% creatine for 14 days. However, creatine did not affect isometric strength after eccentric muscle contractions.
As shown in Tables 1 and 2, several studies have reported that creatine attenuates exercise-induced muscle damage (Bassit et al., 2010; Cooke et al., 2009; Rosene et al., 2009; Veggi et al., 2013). Cooke et al. (2009) showed that healthy males ingesting creatine (loading period: 0.3 g/kg/day, four servings/day, 5 days; maintenance period: 0.1 g/kg/day, one serving/day, 14 days) beginning 5 days before exercise until 14 days after exercise improved maximal isometric strength and decreased CK compared with those who consumed a carbohydrate placebo only. Bassit et al. (2010) also reported that ingesting 20 g/day creatine over 5 days decreases CK and LDH after a triathlon competition.
Rosene et al. (2009) reported the acute (20 g/day, 7 days) and chronic (6 g/day, after 7 days followed for 23 days) effects of creatine on exercise-induced muscle damage. This study demonstrated that chronic ingestion of creatine effectively increased maximal isometric strength after resistance exercise. Veggi et al. (2013) suggested that taking 20 g/day creatine for 6 days between the first and the second exercise phase contributed to decreased muscle soreness, inhibited the elevation in CK, and enhanced of range of motion. Two studies (Rosene et al., 2009; Veggi et al., 2013), suggested that taking creatine may increase the “repeated bout effect” after initial exercise-induced muscle damage. The repeated bout effect is protective against subsequent muscle damage through neural, mechanical, and cellular adaptations after exercise (McHugh, 2003).
However, several studies suggested that creatine had no benefit on exercise-induced muscle damage (McKinnon et al., 2012; Rawson et al., 2001, 2007). Rawson et al. (2001) demonstrated that creatine (20 g/day) taken for 5 days before and after exercise does not change the levels of muscle damage markers after exercise between subjects taking creatine and a placebo. Rawson et al. (2007) reported that creatine (loading period: 0.3 g/kg/day, three servings/day, 5 days; maintenance period: 0.03 g/kg/day, one serving/day, 5 days) does not change muscle damage marker levels after exercise. Similarly, McKinnon et al. (2012) reported that taking creatine (loading dose: 40 g, two servings/day, 5 days; maintenance period: 10 g, two servings/day, 5 days) had no effect on exercise-induced muscle damage. These conflicting results may be partly explained by differences in exercise protocols used in the studies.
POTENTIAL MECHANISMS OF CREATINE ON EXERCISE-INDUCED MUSCLE DAMAGE
A number of potential mechanisms explain the effect of creatine on exercise-induced muscle damage, including the inflammatory response, oxidative stress, calcium homeostasis, and satellite cells activities in damaged muscle (Fig. 1). The first potential mechanism of creatine is that it reduces the inflammatory response after exercise-induced muscle damage. Santos et al. (2004) demonstrated that 20 g/day creatine for 5 days before 34 male marathon runners raced significant reduced LDH, prostaglandin E2 (PGE2), and tumor necrosis factor-a (TNF-α) after the 30 km race. These results agree with those of several studies. Bassit et al. (2008) reported that 11 male triathletes who ingested 20 g/day creatine for 5 days prior to a half-Ironman competition had significant decreases in TNF-α, interferon-α (INF-α), interleukin-1β (IL-1β), and PGE2 after the competition compared to those in the placebo group. Deminice et al. (2013) also reported that ingesting 0.3 g/kg creatine for 7 days abolishes the increase in TNF-α after a repeated running-based anaerobic test. PGE2 and TNF-α facilitate the inflammatory response and pain sensation after exercise-induced muscle damage (Tidball, 2005).
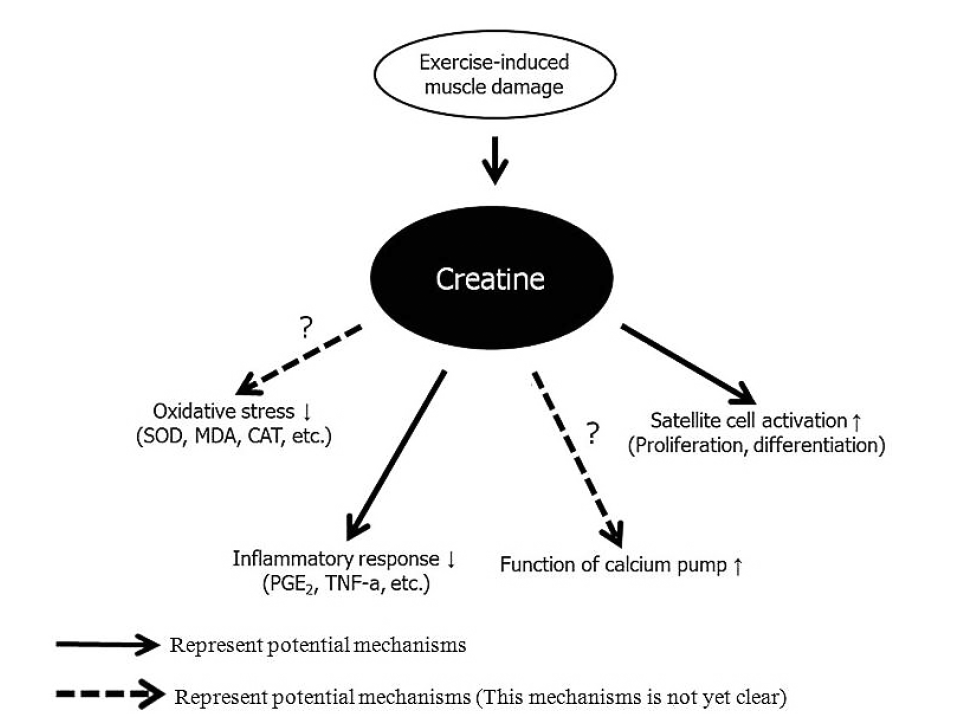
Potential mechanisms of creatine on exercise-induced muscle damage. SOD, Superoxide dismutase; MDA, malondialdehyde; CAT, catalase; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-α.
Interestingly, all three studies showed a decrease in the inflammatory response (Bassit et al., 2008; Deminice et al., 2013; Santos et al., 2004). Santos et al. (2004) particularly showed a decrease in LDH and inflammation. The inflammatory response is associated with markers of sarcolemma damage (Kanda et al., 2013). Kanda et al. (2013) reported a positive correlation between neutrophil migratory activity and myoglobin after exercise. These results indicate that the reduction of inflammatory response factors by creatine may decrease disruption of sarcolemma due to exercise-induced muscle damage. In addition, Bassit et al. (2010) reported that taking creatine (5 g/kg/day, 5 days) significant decreases outflux of intracellular enzymes after continuous muscle contraction. In contrast, Silva et al. (2013) found that ingesting creatine (300 mg/kg/day, 15 days) does not significantly reduce inflammatory response markers, such as TNF-α, IL-1β, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in mice.
The second potential creatine mechanism is diminished oxidative stress (Lawler et al., 2002; Rahimi, 2011). Lawler et al. (2002) reported the first evidence for the antioxidant capacity of creatine. Deminice and Jordao (2012) found that ingesting 2% creatine during the 28 days before acute exercise decreases thiobarbituric acid-reactive substances (TBARS) and lipid hydroperoxides but increases the glutathione (GSH) and glutathione disulfide (GSSG) ratio and total antioxidant capacity. However, these studies were limited to cultured cells models, and animals. According to a human study by Rahimi (2011), taking 20 g/day creatine for 7 days decreases malonyldialdehyde (MDA) and 8-hydroxy-2-deoxyguanosine (8-OHdG) levels after resistance exercise compared to those taking a placebo. In contrast, several studies have reported that creatine does not decrease oxidative stress after exercise-induced muscle damage (Deminice et al., 2013; Silva et al., 2013). Therefore, this mechanism remains unclear.
Another creatine mechanism is regulation of calcium homeostasis. Impaired sarcoplasmic reticulum due to muscle damage may increase calcium concentrations in the cytosol, causing secondary muscle damage (Beaton et al., 2002). Creatine assists in maintaining the sarcoplasmic reticulum calcium pump function by phosphorylating ADP to ATP, which decreases cytosolic calcium levels (Cooke et al., 2009; Korge et al., 1993). Minajeva et al. (1996) suggested that increasing muscle PCr accelerates ATP homeostasis, leading to reduced secondary damage due to an increase in calcium concentration. However, this hypothesis needs further research.
Finally, creatine has been associated with satellite cells or so-called “muscle stem cells” (Olsen et al., 2006; Safdar et al., 2008). Satellite cells play a critical regenerating role after muscle damage (Paulsen et al., 2012). Olsen et al. (2006) demonstrated that ingesting creatine (loading period: 24 g/day, 6 g/serving, four servings/day, 7 days; maintenance period: 6 g/day, one serving/day, 15 weeks) and performing resistance exercise increases the number of satellite cells and myonuclei concentration in human muscle. In addition, Safdar et al. (2008) reported that taking creatine (loading period: 20 g/day, 10 g/serving, two servings/day, 3 days; maintenance period: 5 g/day, one serving/day, 7 days) promotes proliferation and differentiation of satellite cells and activate cytoskeletal remodeling genes. In contrast, Crassous et al. (2009) reported that creatine has no effect on regenerating muscle after damage. However, this study damaged muscle using notexin, not exercise.
CONCLUSIONS
Creatine may be a useful dietary supplement for preventing muscle damage and facilitating recovery from high-intensity exercise, which is applicable to the sports rehabilitation field. However, several mechanisms of how creatine prevents exercise-induced muscle damage need to be examined in future well-designed studies.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.

