Music application alleviates short-term memory impairments through increasing cell proliferation in the hippocampus of valproic acid-induced autistic rat pups
Article information
Abstract
Autism is a neurodevelopmental disorder and this disorder shows impairment in reciprocal social interactions, deficits in communication, and restrictive and repetitive patterns of behaviors and interests. The effect of music on short-term memory in the view of cell proliferation in the hippocampus was evaluated using valproic acid-induced autistic rat pups. Animal model of autism was made by subcutaneous injection of 400-mg/kg valproic acid into the rat pups on the postnatal day 14. The rat pups in the music-applied groups were exposed to the 65-dB comfortable classic music for 1 hr once a day, starting postnatal day 15 and continued until postnatal day 28. In the present results, short-term memory was deteriorated by autism induction. The numbers of 5-bromo-2′-deoxyridine (BrdU)-positive, Ki-67-positive, and doublecortin (DCX)-positive cells in the hippocampal dentate gyrus were decreased by autism induction. Brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) expressions in the hippocampus were also suppressed in the autistic rat pups. Music application alleviated short-term memory deficits with enhancing the numbers of BrdU-positive, Ki-67-positive, and DCX-positive cells in the autistic rat pups. Music application also enhanced BDNF and TrkB expressions in the autistic rat pups. The present study show that application of music enhanced hippocampal cell proliferation and alleviated short-term memory impairment through stimulating BDNF-TrkB signaling in the autistic rat pups. Music can be suggested as the therapeutic strategy to overcome the autism-induced memory deficits.
INTRODUCTION
Autism is a neurodevelopmental disorder and this disorder shows impairment in reciprocal social interactions, deficits in communication, and restrictive and repetitive patterns of behaviors and interests (Vernazza-Martin et al., 2005). This disorder is generally diagnosed between 2–4 yr of age via clinician observation and parental questionnaires, although signs and symptoms are often observed at younger age (Landa, 2008).
Prenatal exposure to teratogenic agents has been suggested as the pathogenic etiology of autism (Vernazza-Martin et al., 2005; Williams et al., 2001). Exposure to anticonvulsants during pregnancy causes one or more aberrant behaviors with the incidence of 36 of 57 (81%) in children (Moore et al., 2000). In particular, valproic acid appears as the risk factor inducing autism spectrum disorder (Bromley et al., 2008).
Hippocampus is an important brain area for the learning process and memory capability, and neuropathological alterations of hippocampus probably account for early symptoms of neuropsychiatric diseases (Balu and Lucki, 2009; Braak et al., 2006). Cell proliferation in the hippocampus is implicated in the hippocampal function, and disturbance of hippocampal cell proliferation is associated with abnormal behaviors (Kim et al., 2013; Lee et al., 2015; Scharfman and Hen, 2007).
For the detection of newly formed neurons, 5-bromo-2′-deoxyridine (BrdU) immunohistochemistry and Ki-67 immunohistochemistry have widely been used (Kim et al., 2006; Lee et al., 2015; Sung et al., 2010). Decrement of these numbers represents suppression of new cell formation (Kim et al., 2013; Sung et al., 2010). Doublecortin (DCX) is a marker of neuronal precursor cells and DCX is also associated with structural plasticity in the adult mammalian brain (Kim et al., 2013). DCX modulates neuronal migration and maturation, as a result, DCX identifies newly formed neurons in the adult hippocampus (Friocourt et al., 2007).
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, and performs its action through binding to its receptor, tyrosine kinase B (TrkB). BDNF-TrkB interaction promotes neuronal survival and differentiation, increases synaptic plasticity, and improves learning ability and memory function (Kim et al., 2012; Kim et al., 2015; Li et al., 2012; Vaynman et al., 2004).
Music therapy has been used for developmentally delayed children, mentally ill adults, atrisk youth, chronic disease patients, geriatric patients, and patients in psychotherapy. It is also recommended as a treatment modality for children with autism (Kemper and Danhauer, 2005). Application of music during pregnancy showed enhancement of neurogenesis in the hippocampus of rat pups and these rat pups also showed enhanced spatial learning memory (Kim et al., 2006). Chaudhury et al. (2010) reported that prenatal auditory stimulation caused morphological and biochemical changes in the hippocampus and enhanced spatial learning ability. In addition, the number of neurons in the auditory nuclei and hippocampus was increased by music application (Sanyal et al., 2013).
In the present study, we evaluated the effect of music application on short-term memory in the view of cell proliferation in the hippocampus using valproic acid-induced autistic rat pups. Step-dawn avoidance task was conducted for the evaluation of short-term memory. Immunohistochemistry for BrdU, Ki-67, and DCX in the hippocampal dentate gyrus was performed for the calculation of cell proliferation. BDNF and TrkB expressions in the hippocampus were evaluated by Western blot analysis.
MATERIALS AND METHODS
Experimental animals and treatments
Male Sprague-Dawley rat pups (25±5 g, 2 weeks old) were used, and the experimental procedures were performed in accordance with the animal care guidelines of the Korean Academy of Medical Sciences and also with the National Institutes of Health. The animals were divided into 4 groups (n=8 in each group): Control group, music-applied group, autism-induced group, autism-induced and music-applied group. One hr before the exposure to music, all rat pups received 50-mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally, once a day, from postnatal day 15 to postnatal day 19.
Induction of autism
To make autism-like animal model, 400-mg/kg valproic acid (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), dissolved in saline at a concentration of 0.1 mL/kg, was subcutaneously injected into the rat pups on the postnatal day 14, as the previous method (Seo et al., 2013; Wagner et al., 2006). Day of birth was recorded as day 0, and all rat pups were labeled for individual identification. The rat pups in the control group and in the music-applied group received subcutaneous injection of saline in the same volume.
Exposure to music
The rat pups in the music-applied groups were exposed to the 65-dB comfortable classic music for 1 hr once a day, starting postnatal day 15 and continued until postnatal day 28, as the previous method (Kim et al., 2006). The rat pups in the control group and in the autism-induced group were left undisturbed.
Step-down avoidance task
Latency in the step-down avoidance task was used for the evaluation of short-term memory, as the previous method (Kim et al., 2013; Sung et al., 2010). The rat pups were placed on a 7×25-cm platform that was 2.5 cm in height and allowed to rest on the platform for 2 min. The platform faced a 42×25-cm grid of parallel 0.1-cm caliber stainless steel bars spaced 1 cm apart. On the postnatal day 28, the animals received 0.3-mA scrambled foot shock for 2 sec immediately upon stepping down. The latency was determined 2 days after the training session. The time interval between the moment when the rats first stepped down and when they placed all four paws on the grid was defined as the latency in the step-down avoidance task. Latency over 300 sec was counted as 300 sec.
Tissue preparation
The rat pups were sacrificed on the postnatal day 30, immediately after step-down avoidance task. The animals were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50-mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100-mM phosphate buffer (pH, 7.4). The brains were dissected and postfixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Forty-micrometer-thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistry
Immunohistochemistry for BrdU was performed, as the previous method (Kim et al., 2006; Lee et al., 2015). The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min. They were then pretreated in 50% formamide-2× standard saline citrate at 65°C for 2 hr, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH, 8.5). Thereafter, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 hr with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were then incubated with avidin-peroxidase complex (1:100; Vector Laboratories) for 1 hr. For visualization, the sections were incubated in 50 mM Tris-HCl (pH, 7.6) containing 0.02% 3,3-diaminobenzidine (DAB), 40-mg/mL nickel chloride, and 0.03% H2O2 for 5 min.
After BrdU-specific staining, differentiation of BrdU-positive cells was determined on the same section using a mouse antineuronal nucleic antibody (1:300; Chemicon International, Temecula, CA, USA). For staining, the sections were incubated in a reaction mixture consisting of 0.02% DAB and 0.03% H2O2 for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted with Permount (Fisher Scientific, New Jersey, NJ, USA).
Ki-67 immunohistochemistry
Immunohistochemistry for Ki-67 was performed, as the previous method (Sung et al., 2010). The sections were then incubated in 0.05 M PBS for 10 min and washed 3 times using 50-mM PBS, then were incubated in 3% H2O2 for 30 min. After that, the sections were incubated overnight with mouse anti-Ki-67 antibody (1:500; Novocastra Laboratories, Newcastle, UK). Next day, the sections were incubated for 1 hr with biotinylated antimouse secondary antibody (Vector Laboratories), subsequently incubated with an avidin–biotin–peroxidase complex (Vector Laboratories) for 1 hr at room temperature. The immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% DAB and 0.01% H2O2 in 50-mM Tris-buffer (pH, 7.6) for approximately 3 min. The sections were then mounted on gelatin-coated glass slides, and the coverslips were mounted using Permount (Fisher Scientific).
DCX immunohistochemistry
DCX immunohistochemistry was performed, as the previous method (Friocourt et al., 2007; Kim et al., 2013). The sections were incubated overnight with a mouse anti-DCX antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then for another 1 hr with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with a Vector Elite ABC kit (Vector Laboratories). The antibody–biotin–avidin-peroxidase complexes were visualized using 0.02% DAB. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and the coverslips were mounted using Permount (Fisher Scientific).
Western blot analysis
BDNF and TrkB expressions were determined by Western blot analysis (Kim et al., 2006; Kim et al., 2015). The hippocampal tissues were homogenized on ice, and lysed in a lysis buffer containing 50-mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (pH, 7.5), 150-mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1-mM ethyleneglycol- bis-(b-aminoethylether)-N,N,N′,N′-tetraacetic acid, 1.5-mM MgCl2·6H2O, 1-mM sodium orthovanadate, and 100-mM sodium flouride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). The protein (30 μg) was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse beta-actin antibody (1:1,000; Santa Cruz Biotechnology), rabbit BDNF antibody (1:500; Santa Cruz Biotechnology), rabbit TrkB antibody (1:1,000; Santa Cruz Biotechnology), were used as the primary antibodies. Horseradish peroxidase conjugated antirabbit antibody for BDNF and TrkB (1:3,000; Vector Laboratories) and horseradish peroxidase-conjugated antimouse antibody for beta-actin (1:2,000; Vector Laboratories) were used as the secondary antibodies. Band detection was performed using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
The numbers of BrdU-positive, Ki-67-positive, and DCX-positive cells in the hippocampal dentate gyrus were counted hemilaterally using Image-Pro Plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). These numbers were expressed as the numbers of cells per mm2 of cross-sectional area of the hippocampal dentate gyrus. For confirming the expressions of BDNF and TrkB, the detected bands were calculated densitometrically using Molecular Analyst, ver. 1.4.1 (Bio-Rad). Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test, and the results are expressed as the mean±standard error of the mean. Significances were accepted at P<0.05.
RESULTS
Effect of music application on the latency in the step-down avoidance task
The present results show that short-term memory was deteriorated by induction of autism and that application of music alleviated autism-induced impairment of short-term memory. In the normal rat pups, music application did not exert any effects on the short-term memory (Fig. 1).
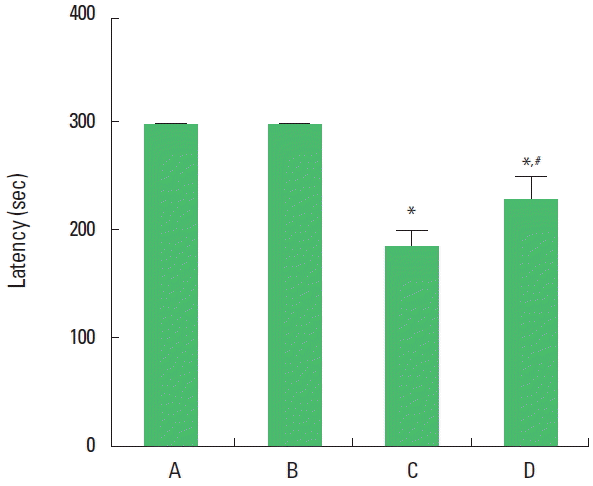
Effect of music application on latency in the step-down avoidance task. The data are represented as the mean±standard error of the mean. A, control group; B, music-applied group; C, autism-induced group; D, autism-induced and music-applied group. *P<0.05 compared to the control group. #P<0.05 compared to the autism-induced group.
Effect of music application on the number of BrdU-positive cells in the hippocampal dentate gyrus
The present results show that the number of BrdU-positive cells was decreased by induction of autism and that application of music increased the number of BrdU-positive cells in the autism-induced rat pups. In the normal rat pups, music application did not exert any effects on the number of BrdU-positive cells (Fig. 2).
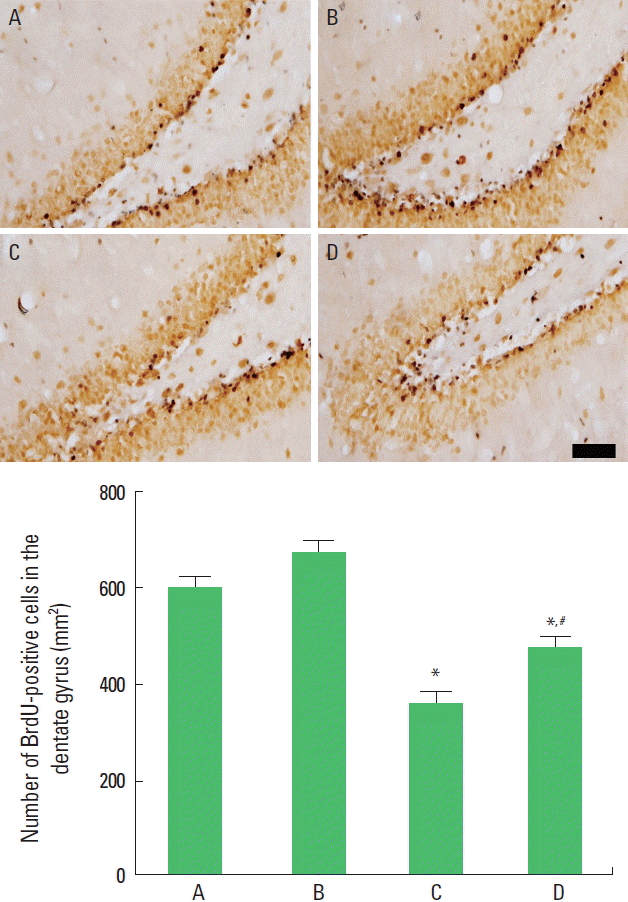
Effect of music application on the number of 5-bromo-2′-deoxyridine (BrdU)-positive cells in the hippocampal dentate gyrus. Upper panel: Photomicrographs of BrdU-positive cells in the hippocampal dentate gyrus (immunohistochemistry). The scale bar represents 50 μm. Lower panel: The number of BrdU-positive cells in each group. The data are represented as the mean±standard error of the mean. A, control group; B, music-applied group; C, autism-induced group; and D, autism-induced and music-applied group. *P<0.05 compared to the control group. #P<0.05 compared to the autism-induced group.
Effect of music application on the number of Ki-67-positive cells in the hippocampal dentate gyrus
The present results show that the number of Ki-67-positive cells was decreased by induction of autism and that application of music increased the number of Ki-67-positive cells in the autism-induced rat pups. In the normal rat pups, music application increased the number of Ki-67-positive cells (Fig. 3).
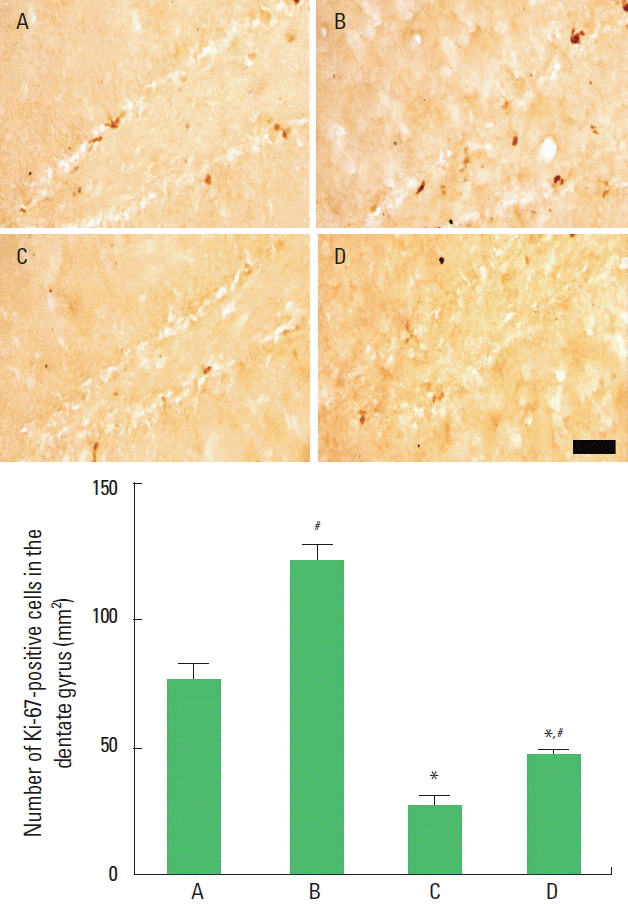
Effect of music application on the number of Ki-67-positive cells in the hippocampal dentate gyrus. Upper panel: Photomicrographs of Ki-67-positive cells in the hippocampal dentate gyrus (immunohistochemistry). The scale bar represents 50 μm. Lower panel: The number of Ki-67-positive cells in each group. The data are represented as the mean±standard error of the mean. A, control group; B, music-applied group; C, autism-induced group; and D, autism-induced and music-applied group. *P<0.05 compared to the control group. #P<0.05 compared to the autism-induced group.
Effect of music application on the number of DCX-positive cells in the hippocampal dentate gyrus
The present results show that the number of DCX-positive cells was decreased by induction of autism and that application of music increased the number of DCX-positive cells in the autism-induced rat pups. In the normal rat pups, music application increased the number of DCX-positive cells (Fig. 4).

Effect of music application on the number of doublecortin (DCX)-positive cells in the hippocampal dentate gyrus. Upper panel: Photomicrographs of DCX-positive cells in the hippocampal dentate gyrus (immunohistochemistry). The scale bar represents 50 μm. Lower panel: The number of DCX-positive cells in each group. The data are represented as the mean±standard error of the mean. A, control group; B, music-applied group; C, autism-induced group; and D, autism-induced and music-applied group. *P<0.05 compared to the control group. #P<0.05 compared to the autism-induced group.
Effects of music application on the expressions of BDNF and TrkB in the hippocampus
The present results show that expressions of BDNF and TrkB were suppressed by induction of autism and that application of music enhanced BDNF and TrkB expressions in the autism-induced rat pups. In the normal rat pups, music application increased BDNF and TrkB expressions (Fig. 5).

Effect of music application on the expressions of brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) in the hippocampus. The data are represented as the mean±standard error of the mean. A, control group; B, control and music-applied group; C, autism-induced group; and D, autism-induced and music-applied group. *P<0.05 compared to the control group. #P<0.05 compared to the autism-induced group.
DISCUSSION
Autistic spectrum disorder is one of the pervasive neurodevelopmental disorders and accompanies with progressive deficits in learning ability, memory capability, and cognitive function (Seo et al., 2013; Theoharis et al., 2013). Presently, latency in the step-down avoidance task was shortened by induction of autism. This indicates that short-term memory was deteriorated in the autistic rat pups.
Many therapeutic strategies were tried to improve communication and social interaction in patients with autistic spectrum disorder, however, the evidences of their effectiveness are not sufficient (Wigram and Gold, 2006; Williams et al., 2001). Music therapy has a long history for the treatment of autistic spectrum disorder, meanwhile emotional, motivational, and interpersonal responsiveness of children with autism were improved by music therapy (Gold, 2011; Wigram and Gold, 2006). Presently, application of music increased latency in the step-through avoidance task in the autistic rat pups, indicating that music alleviated autism-induced short-term memory impairment.
Music is also known to improve spatial learning ability through increasing neurogenesis (Kim et al., 2006). Suppression of new cell formation in the hippocampus is closely associated with memory impairment in aging, dementia, epilepsy, and other brain inflammatory conditions (Baek et al., 2012; Kim et al., 2010; Sung et al., 2010). Age-induced memory impairment is known to be related with suppression of new cell formation in the hippocampus (Kim et al., 2010) and depression is also accompanied with inhibition of neurogenesis in the hippocampus (Sung et al., 2010). Presently, the numbers of BrdU-positive and Ki-67-positive cells in the hippocampus were suppressed in the autistic rat pups. Application of music increased the number of BrdU-positive and Ki-67-positive cells in the autistic rat pups.
DCX is widely used as the marker reflecting the level of neurogenesis and its modulation (Friocourt et al., 2007). DCX is a microtubule associated with differentiating and migration of neurons (Gong et al., 2010). Presently, DCX expression in the hippocampus was suppressed, in contrast, application of music enhanced DCX expression in the autistic rat pups.
BDNF is implicated in the neuroprotective action and memory consolidation. Over-expression of BDNF and its receptor TrkB enhanced memory function through increasing neurogenesis, whereas inhibition of BDNF and TrkB caused memory impairment through suppressing neurogenesis (Alonso et al., 2002; Lee and Son, 2009). Suppression on BDNF-TrkB signaling is associated with the inhibition of cell proliferation in the hippocampal dentate gyrus (Kim et al., 2012). Application of music during pregnancy alleviated memory impairment with increasing of BDNF expression in rat pups (Kim et al., 2006). Presently, expressions of BDNF and TrkB in the hippocampus were decreased, in contrast, application of music increased BDNF and TrkB expressions in the autistic rat pups.
Our study shows that music exerted enhancing effect on hippocampal cell proliferation in the autistic rats. Application of music also exerted alleviating effect on autism-induced short-term memory impairment through stimulating BDNF-TrkB signaling.
Based on the present study, suppression of cell proliferation in the hippocampus might be one of the etiologic factors causing autism-induced memory impairments. Music can be suggested as the therapeutic strategy to overcome the autism-induced memory deficits. These effects of music could be ascribed to the enhancing effect of music on cell proliferation in the hippocampus through BDNF-TrkB signaling pathway.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.