Treadmill exercise facilitates recovery of locomotor function through axonal regeneration following spinal cord injury in rats
Article information
Abstract
Spinal cord injury (SCI) disrupts both axonal pathways and segmental spinal cord circuity, resulting in permanent neurological deficits. Physical exercise is known to increase the expression of neurotrophins for improving the injured spinal cord. In the present study, we investigated the effects of treadmill exercise on locomotor function in relation with brain-derived neurotrophic factor (BDNF) expression after SCI. The rats were divided into five groups: control group, sham operation group, sham operation and exercise group, SCI group, and SCI and exercise group. The laminectomy was performed at the T9–T10 level. The exposed dorsal surface of the spinal cord received contusion injury (10 g × 25 mm) using the impactor. Treadmill exercise was performed 6 days per a week for 6 weeks. In order to evaluate the locomotor function of animals, Basso-Beattie-Bresnahan (BBB) locomotor scale was conducted once a week for 6 weeks. We examined BDNF expression and axonal sprouting in the injury site of the spinal cord using Western blot analysis and immunofluorescence staining. SCI induced loss of locomotor function with decreased BDNF expression in the injury site. Treadmill exercise increased the score of BBB locomotor scale and reduced cavity formation in the injury site. BDNF expression and axonal sprouting within the trabecula were further facilitated by treadmill exercise in SCI-exposed rats. The present study provides the evidence that treadmill exercise may facilitate recovery of locomotor function through axonal regeneration via BDNF expression following SCI.
INTRODUCTION
Spinal cord injury (SCI) is often accompanied with tissue damage, limited endogenous repair, and loss of motor, sensory, and autonomic function, and sometimes results in long-term and severe disability. In the injured spinal cord, there are cell death, severing axons, demyelination, inflammation, and formation of cystic cavities (Sekhon and Fehlings, 2001). The injured spinal cord lacks the intrinsic capability to replace the cavity in the tissue therefore there is a limited capability for axonal regeneration after injury (Tang et al., 2007).
In the central nervous system (CNS), there is a limited capacity for axonal regeneration after injury. The failure of neuronal regeneration may be explained in part by the lack of neurotrophins required for axon outgrowth and guidance (Goldshmit et al., 2004). To promote axonal regeneration after CNS injury, suitable environment conditions for regeneration are critical. One of the important factors to create favorable environmental conditions is neurotrophin (Han et al., 2009). Of these, brain-derived neurotrophic factor (BDNF) is a plasticity-related molecule implicated in neuronal activity, survival, and remodeling. BDNF, transferred to the injured spinal cord, promoted regenerative growth (Diener and Bregman, 1994) and stimulate hindlimb stepping (Jakeman et al., 1998). BDNF mediated a variety of essential morphological changes at neuronal levels including dendritic arborization (Liu et al., 2009), axonal and dendritic remodeling (Yacoubian and Lo, 2000), synaptogenesis (Liu et al., 2009), and synaptic efficacy (Sallert et al., 2009).
Schwann cells generate neurotrophic factors, cell adhesion molecules, and extracellular matrix molecules which promote axonal growth (Oudega and Xu, 2006). In the Schwann cells of motor axons in particular, this neurotrophic factor is also upregulated several folds (Höke et al., 2006). Purified adult rat Schwann cells, injected into the contused rat spinal cord, decreased tissue loss after injury (Azanchi et al., 2004). Schwann cells grafting into a contusion lesion promoted myelination and supraspinal and spinal axon regeneration and improved hindlimb motor function (Takami et al., 2002).
Physical exercise facilitated the expression of many genes and increased trophic factor production in the brain (Neeper et al., 1995) and spinal cord (Gómez-Pinilla et al., 2001). Ying et al. (2003) showed that physical activity increased the expression of BDNF in the intact and injured spinal cord. Treadmill training improved locomotor function in chronic and complete spinalized cats (de Leon et al., 1998), as well as in incomplete paraplegic patients (Calancie et al., 1994). Fouad et al. (2000) noted that treadmill training improved behavioral recovery after spinal cord partially transection in rats. Step training facilitated locomotor recovery after complete spinal lesions (Frigon and Rossignol, 2006). Physical exercise induced improvement of motor ability and enhanced BDNF expression, thereby contributing to neuronal integrity (Macias et al., 2009). In addition, forced treadmill exercise improved movement pattern, increased axonal growth and sprouting proximal to the lesion site, increased synaptic formation, and suppressed muscle atrophy after spinal cord hemisection (Goldshmit et al., 2008).
The possibility that physical exercise may promote functional recovery after SCI has been suggested (Edgerton et al., 2004; Frigon and Rossignol, 2006). However, the underlying mechanisms of treadmill exercise on SCI have not been clarified. In the present study, we investigated the effect of treadmill exercise on functional recovery of locomotion in relation with the expression of BDNF after spinal cord contusion injury.
MATERIALS AND METHODS
Animals and treatments
Forty adult male Sprague-Dawley rats (180±10 g, 6 weeks old, n=40) were used in this study. The rats were individually housed in plastic home cages under controlled temperature (20°C±2°C) and a light-dark cycle consisting of 12 hr of light and 12 hr of darkness (lights on from 07:00 a.m. to 19:00 p.m.). Food and water were made available ad libitum. The rats were divided into five groups: control group, sham operation group, sham operation and exercise group, SCI group, and SCI and exercise group (n=8 in each group). This study was performed in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences.
Surgical procedures
The rats were anesthetized with chloral hydrate (500 mg/kg, intraperitoneally), and a laminectomy was performed at the T9–T10 level, exposing the cord beneath without disrupting the dura. The spinous processes of T8–T11 were then clamped to stabilize the spine, and the exposed dorsal surface of the cord was subjected to contusion injury using the New York University Impactor System (NYU impactor, New York, NY, USA). The moderate contusion was created by dropping a 10 g rod (2.5 mm in diameter) from a height of 12.5 mm onto the exposed cord. Muscles and skin were then sutured. Urinary bladder was emptied twice a day for one week and then thereafter necessary. The rats in the sham operation group and in the sham operation and exercise group received T10 laminectomy without weight-drop contusion injury.
Treadmill exercise protocol
One week after surgery, the rats in the exercise groups were trained to walk on the treadmill 6 days per a week for 6 weeks. When no stepping of the hindlimb occurred in response to the moving treadmill and the stepping of the forelimb, it was elicited by manual stimulation of the perineum. The training session consisted of four times for 3-min walking during first week, and 6 times for 5-min walking from second week to 6th week, at a speed of 6 m/min with 5-min resting time between each session. In order to evaluate the locomotor function of all animals, Baseline Basso-Beattie-Bresnahan (BBB) locomotor scale was determined once a week.
BBB locomotor scale
Functional recovery was assessed using the BBB locomotor scale, once a week for six weeks. BBB locomotor scale is a standard method for assessing hindlimb locomotion in open field (Yu and Geddes, 2007). Open field locomotion was evaluated by using the 21-point BBB locomotion scale. The rats were placed in an open field (80 cm×130 cm×30 cm) with a pasteboard covered nonslippery floor. In each testing session, the animals were observed individually for 3 min. The scoring range from 0 (flat paralysis) to 21 (normal gait) was graded by features of limb movement, paw placement, gait, and coordination.
Tissue preparation
To begin the sacrificial process, the animals were fully anesthetized using Zoletil 50 (10 mg/kg intraperitoneally; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rats were transcardially perfused with 50-mM phosphate-buffered saline (PBS) and subsequently fixed with freshly prepared 0.5-M phosphate buffer (pH, 7.4) containing 4% paraformaldehyde. Spinal cords were removed and fixed in the same fixative overnight and then transferred into a 30% sucrose solution for cryoprotection. Serial sagittal and cross sections of 20-μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany).
Hematoxylin and eosin staining
For the measuring the volume of cavities in the spinal cord, sagittal and cross sections were stained with hematoxylin and eosin. The slides were dipped into Mayer’s hematoxylin for 30 sec, and then rinsed with tap water until clear. They were then dipped in eosin for 30 sec and again rinsed with water. The slides were dipped twice in 95% ethanol, twice in 100% ethanol, and then twice in 100% xylene. The slides were mounted and examined by a light microscope (Olympus, Tokyo, Japan).
Immunofluorescence
For immunofluorescence, the sections were washed 3 times with PBS, and then exposed to 0.1% bovine serum albumin solution containing 0.1% Tween 20 in PBS for 4 hr at room temperature. Next, sections were incubated overnight with a solution containing primary antibodies as follows: antiglial fibrillary acidic protein monoclonal antibody (1:400, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for astrocyte, anti-neurofilament rabbit polyclonal antibody (1:400, Sigma Chemical Co., St. Louis, MO, USA) for axons, anti-S100β cell monoclonal antibody (1:400, Cosmo Bio Co., Tokyo, Japan) for Schwann cells, and anti-BDNF rabbit polyclonal antibody (1:400, Santa Cruz Biotechnology) for BDNF. After washing, the sections were incubated 2 hr with secondary antibodies as follows: fluorescein isothiocyanate anti-mouse secondary antibody (1:400, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for neurofilament and Schwann cells or CY3 anti-rabbit secondary antibody (1:800, Jackson ImmunoResearch Laboratories) for astrocyte and BDNF. The sections were mounted and examined by a fluorescence microscope (Nikon Eclipse 50i, Nikon Inc., Melville, NY, USA).
Western blot analysis
Protein extracts from spinal cord tissue were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein separation was performed using a 10% polyacrylamide with 0.05% bis-acrylamide. Proteins were then transferred to nitrocellulose and the blots were probed with anti-BDNF rabbit polyclonal antibody (1:1,000, Santa Cruz Biotechnology) and anti-β-actin mouse monoclonal antibody (1:3,000, Santa Cruz Biotechnology). Peroxidase anti-rabbit IgG (1:5,000, Vector Laboratories, Burlingame, CA, USA), and peroxidase anti-mouse IgG (1:5,000, Vector Laboratories) were used as a secondary antibodies. Immunoreactivity was detected by enhanced chemiluminescence (Santa Cruz Biotechnology). Film autoradiograms were exposed from 5 to 15 min.
Data analysis
Differences between groups were evaluated using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) by the one-way analysis of variance followed by Duncan post hoc test. All values are expressed as the mean±standard error of the mean. Statistically significant differences were established at P<0.05.
RESULTS
Treadmill exercise promoted locomotor function after SCI
The BBB scores are presented in Fig. 1. In the present study, the BBB score was significantly decreased after SCI, but treadmill exercise increased BBB score in the SCI rats after 4 weeks (P< 0.05). Functional recovery of hindlimb was facilitated by treadmill exercise.
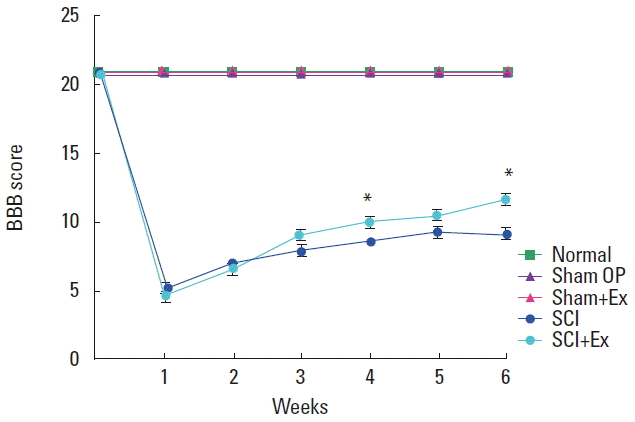
Effect of treadmill exercise on the locomotor function. BBB, Basso-Beattie-Bresnahan; Normal, control group; Sham OP, sham operation group; Sham+Ex, sham operation and exercise group; SCI, spinal cord injury group; SCI+Ex, SCI and exercise group. Values are presented as mean±standard error of the mean. *P<0 .05 compared to the SCI group.
Treadmill exercise decreased cavity formation after SCI
The size of the cavity in the sagittal section of spinal cord was 35.56%±2.48% in the SCI group and 1.84%±0.62% in the SCI and exercise group. The size of the cavity in the cross section of spinal cord was 16.67%±2.09% in the SCI group and 3.37% ±0.83% in the SCI and exercise group (Fig. 2). After SCI, cavity formation was significantly decreased by treadmill exercise (P< 0.05).

Effect of treadmill exercise on the cavity formation in the spinal cord. (a) Upper panel: Photomicrographs of cavity formation in the sagittal section of spinal cord. Arrow indicates cavity. Lower panel: Relative cavity formation in the spinal cord. (b) Upper panel: Photomicrographs of cavity formation in the cross section of spinal cord by hematoxylin and eosin staining. Upper scale represents 100 μm. Lower scale represents 50 μm. Lower panel: Relative cavity formation in the spinal cord. Sham OP, sham operation group; Sham+Ex, Sham operation and exercise group; SCI, spinal cord injury group; SCI+Ex, SCI and exercise group; A, control group; B, sham operation group; C, sham operation and exercise group; D, spinal cord injury (SCI) group; E, SCI and exercise group. Values are presented as mean±standard error of the mean. *P<0 .05 compared to the control group.
Treadmill exercise increased Schwann cells migration and sprouting axons after SCI
We observed the effect of treadmill exercise on the Schwann cells migration after SCI by immunostaining for S100β, which is a marker for Schwann cells (Fig. 3). The expression of S100β-positive cells was increased in the SCI and exercise group compared to the SCI group (P<0.05). Next, to evaluate the axonal sprouting, immunostaining for NF-200 was performed (Fig. 4). Treadmill exercise further increased the number of sprouting axons around the cavity of the injured spinal cord compared with the rats in the SCI group (P<0.05).

Effect of treadmill exercise on Schwann cells proliferation into the cavity of the injured spinal cord. (A) Photomicrographs of cells stained for glial fibrillary acidic protein (GFAP) and S100β in the injured spinal cord following treadmill exercise. (B) Relative intensity of S100β-positive cells in the spinal cord. SCI, spinal cord injury group; SCI+Ex, SCI and exercise group. *P<0.05 compared to the SCI group.

Effects of treadmill exercise on axonal regeneration into the cavity of the injured spinal cord. (A) Photomicrographs showing the expression of glial fibrillary acidic protein (GFAP) and NF-200 in the injured spinal cord following treadmill exercise. (B) Relative intensity of NF-200-positive cells in the spinal cord. SCI, spinal cord injury group; SCI+Ex, SCI and exercise group. *P<0.05 compared to the SCI group.
Treadmill exercise enhanced the expression of BDNF after SCI
The expression of BDNF (14 kDa) in the control group was used as the control value of at 1.00. The expression of BDNF protein was 0.92±0.46 in the sham operation group, 1.93±0.13 in the sham operation and exercise group, 0.49±0.23 in the SCI group, and 1.03±0.24 in the SCI and exercise group (Fig. 5). Treadmill exercise increased BDNF expression in the SCI rats (P<0.05).
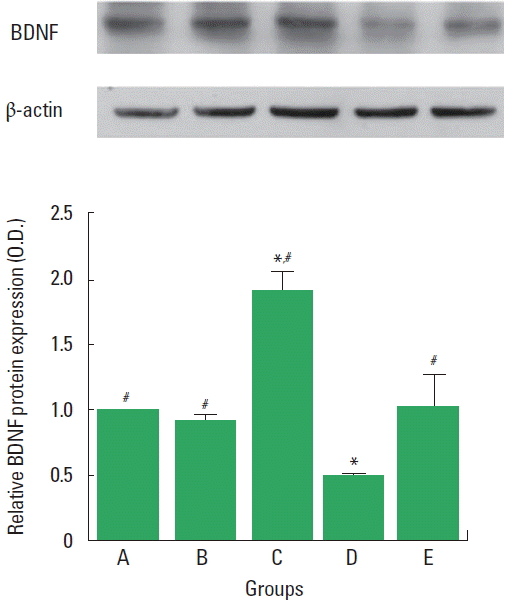
Effect of treadmill exercise on brain-derived neurotrophic factor (BDNF) level in the spinal cord. Upper panel: Representative expression of the protein level of BDNF and β-actin in the spinal cord. Lower panel: Relative BDNF expression in the spinal cord. A, control group; B, sham operation group; C, sham operation and exercise group; D, spinal cord injury (SCI) group; E, SCI and exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the control group. #P<0.05 compared to the SCI group.
Treadmill exercise facilitated axonal sprouting by BDNF expression
Staining intensity of the BDNF within the caudal region was increased by treadmill exercise in the SCI rats. In addition, merged image analysis showed that a majority of BDNF signals was overlapped with that of NF-200 in caudal region of the SCI and exercise group (Fig. 6).
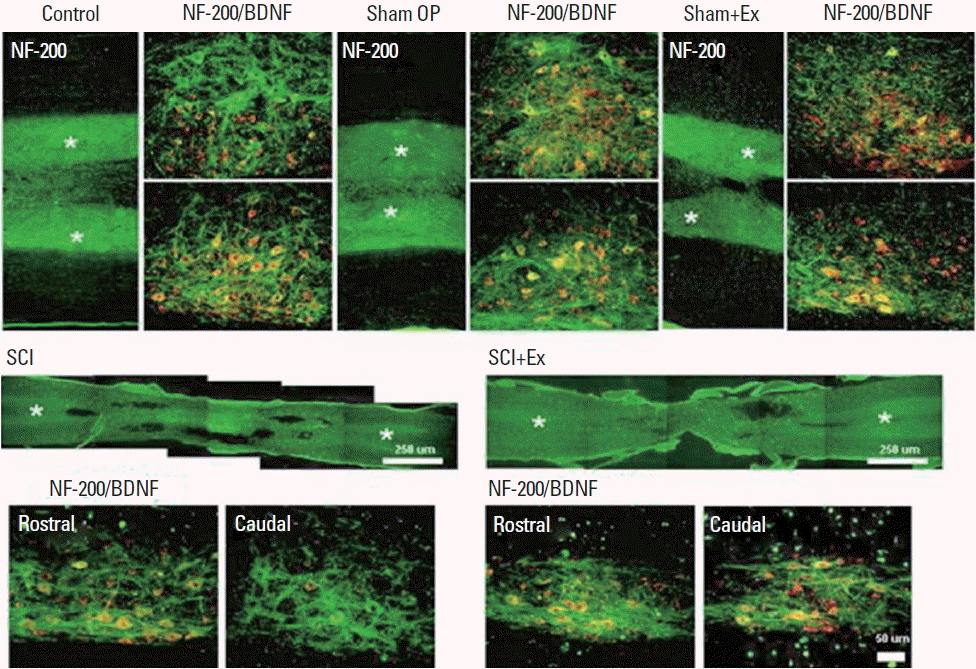
Effect of treadmill exercise on axonal sprouting around injury site by brain-derived neurotrophic factor (BDNF) expression. Photomicrographs of cells stained for BDNF and NF-200 in the injured spinal cord following treadmill exercise. Sham OP, sham operation group; Sham+Ex, sham operation and exercise group; SCI, spinal cord injury group; SCI+Ex, SCI and exercise group.
As shown Fig. 7, when the expression of BDNF in the control group was set as 1.00, the expression of BDNF was 0.48±0.06 in the rostral region, 0.02±0.01 in the epicenter region, and 0.03±0.01 in the caudal region in the SCI group. In the SCI and exercise group, the expression of BDNF was 0.97±0.23 in the rostral region, 1.39±0.32 in the epicenter region, and 1.41±0.38 in the caudal region. The expressions of BDNF in the epicenter and caudal regions were significantly higher in the SCI and exercise group than in the SCI group (P<0.05). Treadmill exercise increased BDNF expression within the caudal region of the injury site following SCI.

Effect of treadmill exercise on brain-derived neurotrophic factor (BDNF) level in each region of the injured spinal cord. Upper panel: Representative expression of the protein level of BDNF and β-actin in each region of the injured cord. Lower panel: Relative BDNF expression in each region of the injured cord. SCI, spinal cord injury group; SCI+Ex, SCI and exercise group. *P<0.05 compared to the SCI group.
DISCUSSION
The effects of physical exercise on functional recovery and expression of neurotrophins have been studied in several models of SCI (Fouad et al. 2000; Goldshmit et al., 2008; Ying et al., 2003). de Leon and Acosta (2006) showed that treadmill training enhancedd locomotor function in spinal injured rats. Forced treadmill exercise improved the pattern of movement after spinal cord hemisection (Goldshmit et al., 2008). In this study, treadmill exercise increased the score of the BBB locomotor scale after SCI. Hindlimb motor disturbance in the rats of SCI and exercise group was not significantly different than that in the SCI group during 3 weeks after injury, However, treadmill exercise increased locomotor function after 4 weeks.
SCI usually undergoes progressive tissue necrosis, which in most cases causes cavity formation (Hofstetter et al., 2002). Oh et al. (2009) reported that the injury cavity was similar between sedentary and treadmill training groups, however, our study showed differed results. Our results showed that induction of SCI caused a cavity formation, in contrast, treadmill exercise reduced the size of this cavity.
Our results also showed that treadmill exercise promoted expression of BDNF in the injured spinal cord. BDNF expression in the injury site was decreased by SCI, but treadmill exercise increased BDNF expression after SCI. In addition, treadmill exercise also increased axonal sprouting. Increment of this BDNF expression in the spinal cord was accompanied with enhancing of axonal sprouting, suggesting that treadmill exercise promoted motor recovery after SCI. In previous study, the levels of BDNF and NT-3 mRNA were reduced by spinal cord hemisection, while exercise returned these mRNA levels near to the normal levels (Ying et al., 2008). Griesbach et al. (2004) reported that postinjury exercise in animals improved recovery from SCI by increasing neurotrophins, such as BDNF and insulin-like growth factor 1. Neurotrophins are important for axonal regeneration after SCI (Cotman and Berchtold, 2002; Hutchinson et al., 2004). As neurotrophins are identified as molecular system activating spinal cord repair, application of exogenous neurotrophins into the CNS has also been tried to induce motor recovery after SCI (Ying et al., 2008). However, this strategy was suggested to decrease the intrinsic capacity of the neural system producing neurotrophins (Ying et al., 2003). For that reason, physical exercise is recommended to promote expressions of endogenous neurotrophins in the injured spinal cord.
Functional recovery is known to be guided by axonal regeneration into the lesion site (Kim et al., 2005). Schwann cell proliferation is functionally correlated with axonal regeneration (Seo et al., 2006). Schwann cells promote axon migration and release neurotrophic factors that further enhance nerve regeneration (Mosahebi et al., 2002). In the present results, Schwann cells aligned into longitudinal array around injury site, relevant to the previous studies (Brook et al., 1994; Weidner et al., 1999). Weidner et al. (1999) reported that Schwann cells grafted to the injured spinal cord lined up into parallel linear arrays in the lesion cavity, providing orientation to subsequently penetrating axons, and promoted and guided axonal growth following SCI. Neurotrophins and Schwann cells can provide trophic and structural support to create a more supportive environment for survival of neurons and regrowth of axons after injury.
Our data showed that treadmill exercise increased the expression of BDNF and also enhaned the sprouting of axons around the injury site. These results suggested that treadmill exercise-induced Schwann cells in the injured spinal cord might be associated with axonal regeneration that promoted functional recovery through increasing BDNF expression. The present study demonstrated that treadmill exercise increased BDNF expression in injury site, and then induced axonal sprouting, thus facilitated functional recovery. The present study provides the evidence that treadmill exercise may facilitate recovery of locomotor function through axonal regeneration via BDNF expression following SCI.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2013S 1A5B5A07048304).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.