Effects of Vojta method on trunk stability in healthy individuals
Article information
Abstract
Vojta reflex locomotion is important to main upright posture through stimulation of breast zone to patient with cerebral palsy. However, application in other diseases is no investigated. So, we determined the effects of stimulation of the breast zone on trunk stability in healthy individuals. Fourteen young healthy adults (7 males and 7 females) voluntarily participated in this study. The subjects were randomly divided into an experimental group (breast zone) and control group (arbitrary point). All groups were stimulated for 5 min on the left and right sides, respectively, for a total 10 times. We used the thickness of the external oblique abdominal muscle (EO), the internal oblique abdominal muscle, the transversus abdominis muscle (TrA), and the rectus abdominis muscles, as well as the area of the diaphragm by using ultrasonography. In the experimental group, the thickness of the TrA significantly increased during stimulation (P<0.05) while the thickness of the EO significantly decreased (P<0.05). Also, the area of diaphragm in inspiration was significantly different (P<0.05). Therefore, stimulation of the breast zone may be effective to improve trunk stability through activation of the TrA muscle and the diaphragm.
INTRODUCTION
Vojta reflex locomotion is a therapeutic approach applied mainly to cerebral palsy that was created and developed by Doctor Vaclav Vojta (Vojta and Peters, 2007). The basic principle of Vojta reflex locomotion is the maintenance of postures through isometric contraction of muscles during point (breast zone) stimulation, thereby ensuring constant patterns of muscle contraction and leading to the stimulation of muscles, joints, ligaments, and tendons. In addition, Vojta reflex locomotion is known to be related to the exteroceptors and the enteroceptors and to become a source of afferent stimulation going into the central nervous system (Vojta, 1977). Vojta reflex locomotion has been reported to activate the trunk muscles and the deep muscles of the spine to regulate trunk stability and increase spinal rotation force, thereby enhancing postural control ability (Son, 2000).
Trunk stability refers to the ability of the core, which is located at the center of gravity of the body, to maintain or regulate body conditions according to changes in the external environment (Hodges, 1999; Zazulak et al., 2008). In particular, since the trunk muscles play the role of a corset that stabilizes the body and the spine, whether the extremities move or not, they are critically important for the maintenance of postures (Akuthota and Nadler, 2004). In addition, the trunk muscles maintain body alignment during sitting and standing postures, and act as supports for performing functions (Leveau and Bernhardt, 1984; Tecklin, 2001). Moreover, the trunk muscles are tonic or postural muscles that play an important role in the segmental stability of the lumbar spine and postural control during whole body exercise (Marshall and Murphy, 2005).
The transversus abdominis muscle (TrA) is activated first among the abdominal trunk muscles before the extremities move. It generates and regulates abdominal internal pressure together with the contraction of the internal oblique abdominal muscle (IO), the diaphragm, and the pelvic floor muscle. It increases the strength of the segmental stability of the lumbar spine to enhance trunk stability (Hodges and Richardson, 1997). In addition, it is known to promote the mobility of the extremities and to play central roles in function movements (Akuthota and Nadler, 2004; Escamilla et al., 2010). The diaphragm, which is involved in respiratory functions and postural control, is an important trunk stabilizing muscle together with the TrA, IO, and the multifidus muscles. They all modulate spinal stiffness, either directly because of the muscle contraction or via increased internal abdominal pressure (Hodges et al., 2007; McGill et al., 1990; Shirley et al., 2003).
In accordance with major trends in rehabilitation, many researchers have become interested in studies related to postural and movement control in patients with damaged brain and trunk stability (Akuthota and Nadler, 2004; Page, 2006). Although Vojta reflex locomotion is a sufficient therapeutic method for the promotion of normal posture control and the direct activation of respiratory muscles in children with cerebral palsy, its application in other diseases has not been well studied (Joo, 2014). Therefore, we sought to examine the effects of the stimulation of the breast zone on trunk stability in normal adults.
MATERIALS AND METHODS
Participants
The subjects were randomly divided into an experimental group (n=7) and a control group (n=7). General characteristics of participants are shown below (Table 1). Informed consent was obtained from all participants after an explanation of their rights and the experimental process. We excluded those participants with orthopedic and neurologic diseases, hypersensitivity, cardiopulmonary disease, open abdominal wounds, or inflammatory disease.
Breast zone stimulation
The breast zone is located between either the 5th and 6th ribs or the 6th and 7th ribs. The starting position for breast zone stimulation was supine with the head turned 30° in the direction of the stimulation, with the extremities lying naturally on the floor. In order to compare the reactions caused by breast zone stimulation, we stimulated an arbitrary point at the intersection of the line 1 cm below the 12th rib and the anterior superior iliac spine in the same starting position as for the breast zone stimulation. In order to maintain a consistent level of stimulation, the intensity was measured using an Algometer (J TECH Medical, Salt Lake City, UT, USA). The intensity was maintained at 1.5 kg/cm2 at the start of stimulation, at 2.5 kg/cm2 when 2 min had passed, and at 3.5 kg/cm2 when 4 min had passed. During the stimulation, the subjects were asked whether they felt pain and any other sensations. All groups were stimulated for 5 min each on the left and right sides, 3 times per week, for a total of 10 rounds of stimulation.
Ultrasonography
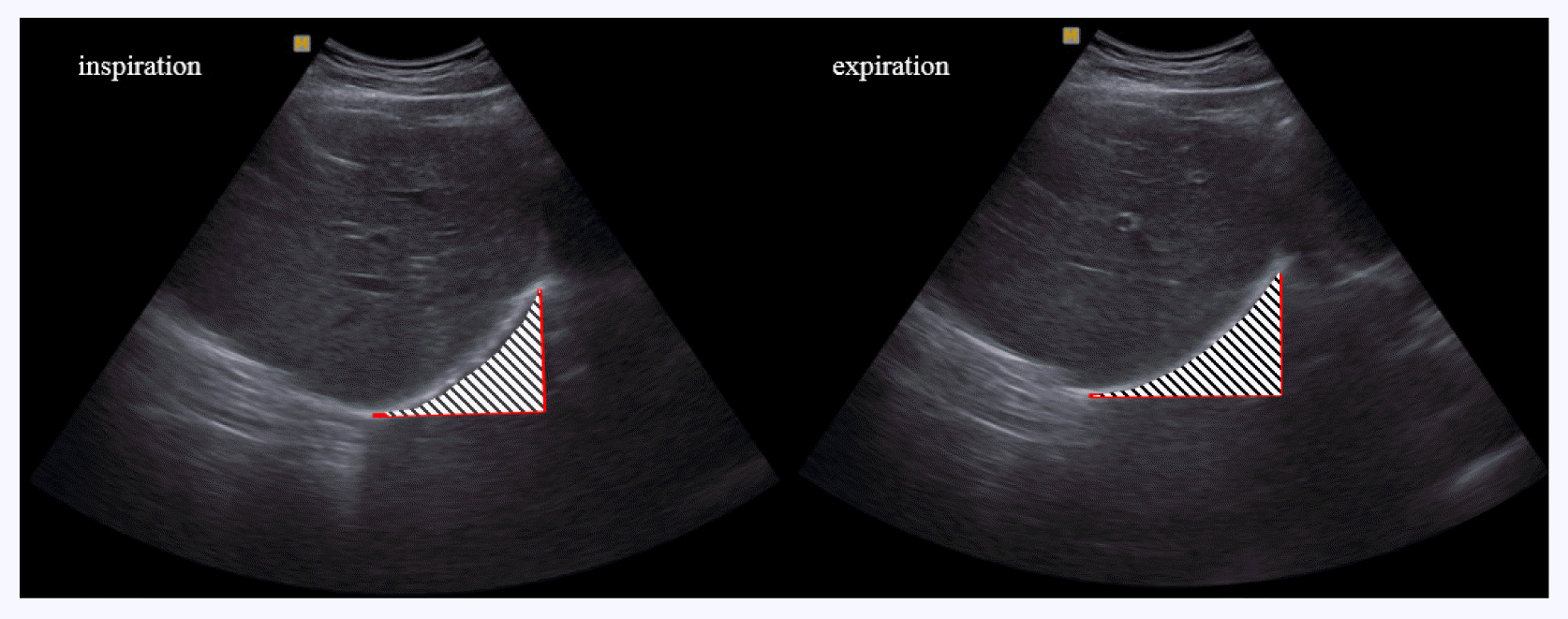
The thicknesses of external oblique abdominal muscle (EO), IO, TrA, rectus abdominis muscle (RA), and area of the diaphragm were measured with ultrasonic image B mode scanning using a diagnostic ultrasonic imaging system (Sonoace X8, Samsung Medison, Seoul, Korea). We used a 7.5-MHz linear probe to measure the EO, IO, TrA, and RA, and a 7.5-MHz sector probe for the diaphragm. All muscles were measured on the right side. For the measurement of the thicknesses of the EO, IO, and TrA, a probe was transversely placed so that the center of the probe contacted the upper part of the iliac crest, centering on the right mid-axillary line. For the measurement of the thickness of the RA, a probe was placed longitudinally at a distance of 2 cm from the navel (Han and Sung, 2015). The thicknesses of the muscles were measured by drawing a vertical line on the center of the image to connect the upper and lower boundaries of the muscle fascia using screen calipers embedded in the ultrasonic imaging system (Fig. 1). The measured values were recorded in cm. The diaphragm was measured during inspiration and expiration after placing a probe on the abdominal wall above the anterior lower ribs on the centerline of the right clavicle in a supine position. We measured the clearly visible semidiaphragm (right dome) when the semidiaphragm was scanned transversely toward the cranium (Testa et al., 2011). The area was quantified by the Image J 1.5 (National Institute of Health, Bethesda, MD, USA) (Fig. 2). The measured values were recorded in pixels. The thicknesses of the EO, IO, TrA, and RA, and the area of the diaphragm during inspiration and expiration were measured before stimulation and after 4 min of stimulation.
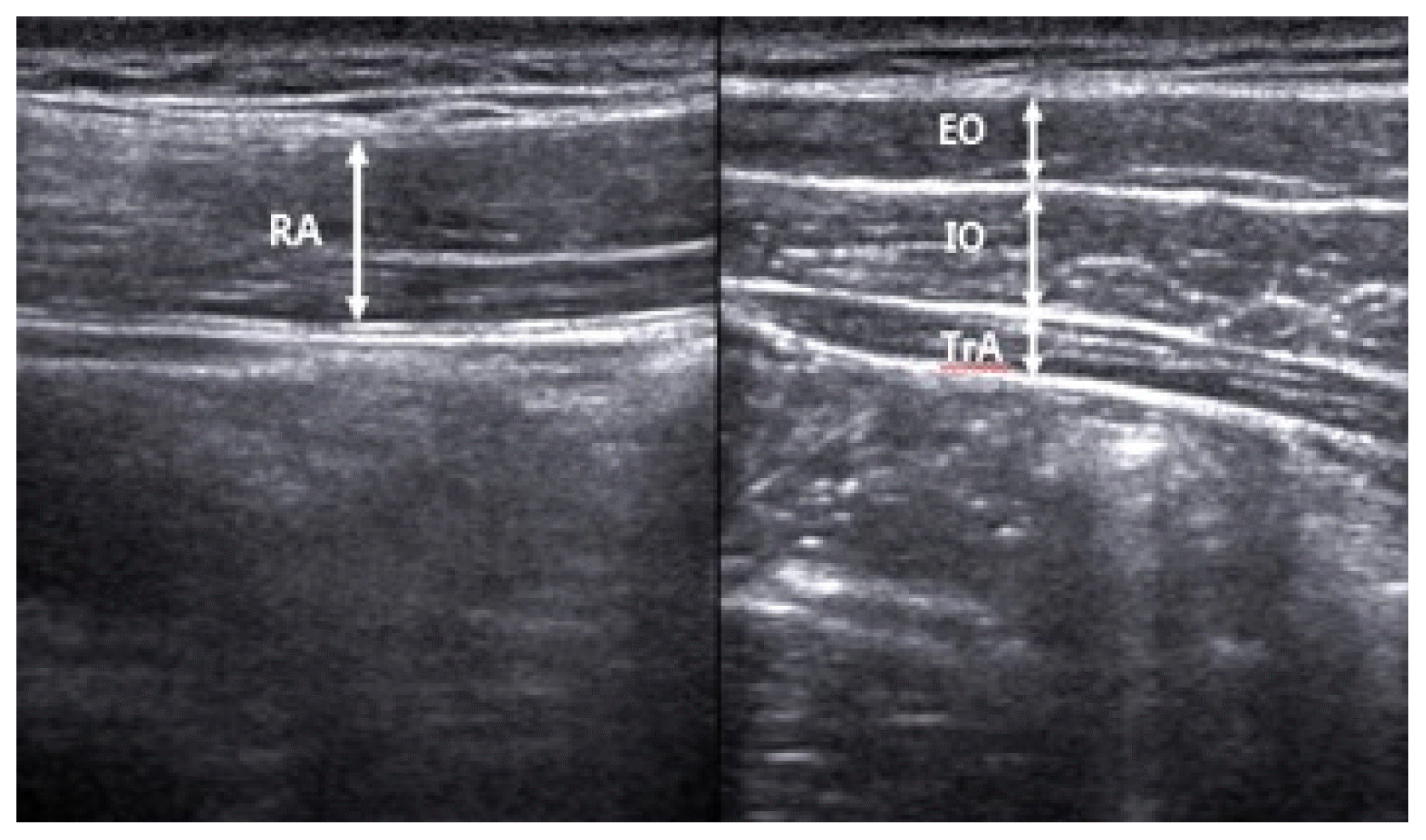
Thickness measurements on the external oblique abdominal muscle (EO), internal oblique abdominal muscle (IO), transversus abdominis muscle (TrA), and rectus abdominis muscle (RA). We investigated the thickness of the muscle before and during stimulation using ultrasonography.
Statistical analysis
All statistical analyses used IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). The results are presented as the mean± standard deviation. Shapiro-Wilk tests were conducted to examine whether individual measurement items were normally distributed. We used Wilcoxon signed ranks tests to analyze changes in the thickness and area of the muscles before and during stimulation. In addition, we used independent t-tests for difference between groups. The significance level was set to P<0.05. All tests were executed twice and the 2 results were averaged.
RESULTS
Muscle thickness
Changes in muscle thickness were analyzed via ultrasonography before and during stimulation in each group. While the muscle thickness of the TrA significantly increased during stimulation compared to the thickness before stimulation in the experimental group (P<0.05), the muscle thickness of the EO significantly decreased (P<0.05). Furthermore, there were no significant differences in the muscle thicknesses of the IO and RA (Table 2) in the experimental group. In the control groups, no significant difference was observed in the muscle thicknesses of the TrA, EO, IO, and RA (P>0.05).
Difference of muscle thickness
We measured difference of muscle thickness before and during stimulation. We confirmed that TrA and EO were significantly difference (P<0.05). However, no significant difference was found in the RA and IO (Table 3).
Area of diaphragm in inspiration and expiration
We examined the area of the diaphragm during inspiration and expiration using ultrasonography before and during stimulation in each group. In the experimental group, significant differences in the area of the diaphragm during inspiration and expiration were found (P<0.05). However, the control group did not show significant differences (P>0.05) (Table 4).
Difference of diaphragm area in inspiration and expiration
We measured difference of diaphragm area in inspiration and expiration before and during stimulation. The changes area of diaphragm in inspiration and expiration were not significant (P> 0.05) (Table 5).
DISCUSSION
Trunk stabilization is generally used for abdominal, lumbar, and pelvic muscle strength training. These muscles are tonic or postural muscles that play an important role in lumbar spine stability and postural control during whole body exercise (Akuthota and Nadler, 2004; Marshall and Murphy, 2005). The muscles related to trunk stabilization are generally divided into global muscles and local muscles. The global muscles include the EO, RA, and the erector spinae muscle. The local muscles include the IO, TrA, and the multifidus muscles (Panjabi, 2003). The activities of the local muscles are highly emphasized to enhance trunk stability (Hodges, 1999; Richardson et al., 2004). In patients with low back pain, the TrA shows abnormal contraction patterns or timing, along with reduced cross-sectional area. The RA and EO indicate excessive activities (Ghamkhar et al., 2011; Hodges and Richardson, 1997; Mannion et al., 2008; O’sullivan et al., 1997; Tsao et al., 2011). Richardson et al. (2002) reported that exercise focused on the activation of local muscles improves the segmental stability of the lumbar spine and dramatically reduces low back pain-related symptoms.
In the present study, although the thickness of the TrA was significantly increased during stimulation in the experimental group, the thickness of the EO was significantly decreased. Kwon et al. (2011) studied the effects of an abdominal hollowing exercise using real-time ultrasound imaging in normal subjects. The results showed that the thickness of the TrA increased, while the thickness of the EO decreased, which is consistent with the results of our study. These results demonstrated that the abdominal hollowing exercise induces a precise recruitment order of trunk muscles, with a strong contraction of the TrA as opposed to the EO, making it a valuable tool for local muscle strength training. Richardson et al. (1999) reported that after the abdominal hollowing exercise was applied to patients with low back pain, the activity of the RA and EO decreased, while the muscle activity of the IO and TrA increased. Henry and Westervelt (2005) proposed that the abdominal hollowing exercise might help patients with musculoskeletal problems such as low back pain because it can induce selective contraction of the local muscles. As previously mentioned, we suggest that stimulation of the breast zone also provokes the selective contraction of local muscles, especially the TrA and IO, to affect trunk stability.
In the present study, we found a considerable change in the area of the diaphragm during inspiration and expiration in the experimental group, but not in the control group. Son (2000) mentioned that stimulation of the breast zone facilitates the active contraction of the diaphragm in infants. The diaphragm primarily acts as a respiratory and postural muscle and is an essential element of spinal stability in the performance of complicated tasks that is also associated with pelvic stability (Frank et al., 2013). Abdominal respiration in patients with low back pain has been shown to affect the trunk stabilization muscles (Kim et al., 2005), and respiratory muscle weakness can cause difficulties with trunk control in neuromuscular disease patients (Lanini et al., 2001). A recent study demonstrated that abnormal diaphragm activation during isometric resistance applied to the extremities puts a greater strain on the ventral region of the spinal column and thus might serve as a fundamental mechanism of chronic low back pain (Frank et al., 2013). In this aspect, we suggested that accurate activation of the diaphragm is critical for trunk stabilization, and the contraction of the diaphragm through breast zone stimulation may positively affect spinal stability to enable better postural control and the improvement of low back pain.
As for the limitations of the present study, although efforts were made to maintain an equal intensity of stimulation, the intensity differed among the subjects. Moreover, since the subjects in our study were selected from among healthy adults, we should confirm our results regarding the contraction and recruitment order of local muscles before and during stimulation of the breast zone in patients with musculoskeletal disease. In addition, since the sample size was too small to generalize the results of the present study, future studies should be conducted with a larger sample size.
In conclusion, stimulation of the breast zone augments activation of the TrA and the diaphragm, and inhibits activation of the EO in normal adults. These findings suggest that breast zone stimulation might strengthen by stimulating the local muscles for trunk stability and postural control in healthy individuals.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.