Skin temperature response to unilateral training measured with infrared thermography
Article information
Abstract
This study aimed to identify the skin temperature (Tsk) behavior to understand the acute cross-effect after unilateral training of lower-limbs. Seventeen healthy young men (weight, 75.2±5.5 kg; height, 1.8±0.1 m; age, 22.5±1.6 years) were divided into two groups: high-trained (n=8) and low-trained (n=9). All participants performed: (a) one-repetition maximum (1RM) testing protocol on the leg press, (b) a unilateral training protocol (4×10 repetitions at 70% of 1RM for leg press and 4×10 repetitions at 50% of 1RM for knee extension). Pre- and posttraining thermal images were recorded. The main results showed that independent of the limb (exercised vs. nonexercised), differences between low- and high-trained were observed for all regions of interest (ROI) except for the anterior knee: posttraining, 30-min and 60-min posttraining in nonexercised limb. The increase of contralateral Tsk was more than 50% on the ROIs corresponding to the exercises muscles 30-min post-training in low-trained but was not so high in high-trained (P<0.05). Low-trained subjects incremented more the Tsk than high-trained in both legs after exercise. In conclusion, we observed an acute contralateral Tsk effect to unilateral training on the Tsk of the nonexercised limb, reliant on the training level of the subject.
INTRODUCTION
Unilateral strength motor activity has been used for rehabilitation purposes (Dragert and Zehr, 2011; Lee and Carroll, 2007). It has been demonstrated that contralateral strength training using training loads about 50% of the maximal voluntary strength, improves by 8% the initial strength values on the untrained limb and the increment on untrained limb correspond to the 25%–50% of the strength increment on the trained side, especially, on the homologous contralateral exercised areas (Carroll et al., 2006). Other studies have shown cross-education effects between 18%–77% of increments on strength values on the contralateral side with training loads higher than 85% (Adamson et al., 2008; Farthing et al., 2007).
Unilateral strength training may also affect the contralateral muscles, because the neural signal is unfolding, stimulating commissural interneurons on the spinal cord, which act on the activation of contralateral motoneurons. Also, the neuromuscular system could provide adaptation to the untrained side improving neural drive to the agonist (Carroll et al., 2006; Lee and Carroll, 2007). A cortex contribution to produce that contralateral effect was presented by Lee et al. (2009). Studies did not show an increase in measures of cross-sectional area of the untrained muscles after unilateral resistance training, or even in the muscle enzymes and muscle fiber types (Beyer et al., 2016). So, muscular mechanism does not seem to be decisive on the registered strength improvement of the contralateral limb (Lee and Carroll, 2007). It is quite evident that peripheral adaptations unlikely appear after contralateral training, due to insufficient stimulus for producing adaptations in the untrained limb. Neural mechanisms are the main responsible for contralateral strength gains after unilateral strength training (Carroll et al., 2006; Fimland et al., 2009). A recent study of Rattey et al. (2006) examined the effects of unilateral exercise and the contralateral fatigue in the nonexercised limb. Results obtained were an 8.7% decrement on the activation of the nonexercised limb. Probably, fatigue in the exercised limb was related to peripheral fatigue mechanisms. Meanwhile, central mechanisms were the factor that greater fatigued the nonexercised limb.
A significant increase during quadriceps muscle activation in a closed chain exercise to maximal fatigue was demonstrated by an increase in the electromyography (EMG) signal. Motor unit activation, and consequential fatigue, is specific to the performed activity of the trained muscle (Pincivero et al., 2006). Cross education can occur in both upper and lower limb muscles, as well as, with several training methods (Lee et al., 2014; Lee and Carroll, 2007; Sariyildiz et al., 2011). The strength gain is confined to the homologous muscle of the opposite untrained limb (Lee and Carroll, 2007; Sariyildiz et al., 2011; Zult et al., 2014), but the fact that the nonhomologous muscle is unaffected by training is important because contralateral effects have major joint specificity and would be greater when larger muscle groups are involved (Magnus et al., 2010) independently of sex or age (Ehsani et al., 2014).
Infrared thermography (IRT), as a noninvasive method, can result useful to control and recording the irradiated energy released from the body through the skin after strength training (Burnham et al., 2006), identify changes in body surface temperature and report the metabolism of active muscles (Chudecka and Lubkowska, 2012). It is widely known that there is a correlation between muscle activation and an increase in the skin temperature of the area adjacent to the muscles involved in the motor action, what is a result of increased metabolism of working muscles (Adamson et al., 2008). The muscles temperature at rest amounts about 36°C, and rises from the beginning to the end of exercise, to 38°C (Adamczyk et al., 2014). The viscoelastic properties of the muscle use the energy stored during deformation for returning to its original shape; however, part of the accumulated energy dissipates as heat, and it relates with muscle activation and contraction (Van Loocke et al., 2008). It has been widely used with rehabilitation purposes, in assessment and reassessment after treatment (Cojocaru et al., 2015; Dimitrijevic et al., 2016; Magalhães et al., 2015).
Thermal asymmetries were correlated with pain intensity in patients with unilateral lumbosacral radiculopathy (Dimitrijevic et al., 2016), automatic detection of diabetic foot complications (Liu et al., 2015) and breast cancer patients detection (de Jesus Guirro et al., 2017). In manual therapy, IRT has also been used to evaluate myofascial trigger points in soft tissues (Dibai-Filho and Guirro, 2015), neuro-musculoskeletal disorders and their dynamics (Cojocaru et al., 2015) and the noncontact, thermal evolution of soft tissues after compression treatment (Magalhães et al., 2015). In view of these data, it seems interesting to relate the influence of the unilateral strength training to the thermal response of both exercised and nonexercised limbs to understand better the physiological foundations of the cross-effect subsequent to this kind of training. We hypothesized that the contralateral training produces an acute thermal cross-effect in nonexercised limbs. In addition, the cross-effect depends on the considered ROI and the level of activity of the subject.
MATERIALS AND METHODS
Approach to the problem
We investigated with IRT acute contralateral Tsk on lower limb recording thermal images of the anterior lower limbs before, during and after unilateral strength training. On the first day of study, participants were engaged in one-repetition maximum (1RM) protocol to calculate the loads of the training day. The next day, after an appropriate acclimatization protocol of 15 min (Fernández-Cuevas et al., 2015), the first thermal image was recorded. Then, the participants performed the warm-up and the second thermal image was collected. Afterwards, they perform the training protocol and thermal images were registered immediately after training, 30 min and 60 min after training.
Subjects
The sample consisted of 17 healthy males, weight: 75.2±5.5 kg (Orbegozo weight scale, model PB2240, Zumarraga, Spain); height, 1.8±0.1 m; age, 22.5±1.6 years. According to levels of strength criteria they were divided into two groups: “high-trained” (1RM in leg press with one leg higher than the body weight: relative strength >1) (n=8; age, 22.3±1.9 years; weight, 76.1±4.3 kg; height, 1.8±0.1 m) and “low-trained” (1RM in leg press with one leg lower than the body weight: relative strength <1) (n=9; age, 22.7±1.1 years; weight, 74.4±6.0 kg; height, 1.78±0.03 m). All the participants signed an informed consent, and this study was approved by the ethical committee of the university in which it was conducted, as a part of the research project “THERMOSPEC” in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Procedures
Previous to the thermography data collection, all the participants were instructed to avoid behaviors that may alter skin temperature (shower, tobacco, alcohol, coffee, etc.) in the night and prior hours before the protocol (Fernández-Cuevas et al., 2015). The thermal images were taken in an acclimated room (temperature, 21.0°C±0.8°C; humidity, 40.3%±1.2%; weather station BAR988HG, Oregon Scientific, Tualatin, OR, USA). For this was used an infrared camera (FLIR T335, FLIR Systems, Wilsonville, OR, USA), according the protocol described by Fernández-Cuevas et al. (2014). The Tsk data were analyzed by a thermographic software analyzer (Thermotracker, PemaGroup, Madrid, Spain). We considered the average values of 10 of the ROIs obtained by the Thermotracker on the thigh muscles and knees areas (Fig. 1).

Considered regions of interest (A) from the software Thermotracker areas (B). 1 & 6, external thigh; 2 & 7, central thigh; 3 & 8, internal thigh; 4 & 9, adductor; 5 & 10, knee (all of them will be named as exercised and nonexercised side).
For the 1RM test and the strength training two machines were used: a leg press (Free Weight Series, Panatta, Apiro, Macerata, Italy) and the knee extension (X Pression Series, Panatta, Apiro, Macerata, Italy). Before the 1RM test, the subjects performed a 5-min warm-up including cycling at 100 W some dynamic stretching for lower body and completed 10 repetitions of back squat only with an Olympic bar. Afterwards, in order to prevent fatigue (Taylor et al., 2012), they had five attempts to achieve the 1RM with each leg in the two considered exercises (leg press and knee extension) with a random order for leg and exercise.
The training sessions included a warm-up of 5-min cycling at 100 W followed by some dynamic lower body stretching and finished with ten repetitions of back squat only with an Olympic bar. The unilateral training exercises and procedures were based on Fernández-Cuevas et al. (2014); choosing leg press and knee extension as exercises. The participants performed 4 sets of 10 repetitions at 70% of 1RM for leg press and 50% of 1RM for knee extension with a 2:2 rhythm (2-sec eccentric and 2-sec concentric). The resting period was 90 sec between sets and 3 min after each exercise. Each exercise was performed only with the strong leg, determined by the leg with higher 1RM in the leg press machine, according to Velotta et al. (2011) and Farthing (2009).
Statistical analysis
Exploratory data analysis was performed for identification and correction of extreme values. Normality and homoscedasticity were tested using the Kolmogorov–Smirnov test and the Bartlett’s criteria, respectively. The analysis of variance (ANOVA) with three factors (training level×strength leg×measurement time) was used to establish the difference between means. For validation of repeated measurements, the Mauchly sphericity test was used and, whenever necessary, Greenhouse–Geisser correction was applied. If a significant difference was observed in the ANOVA, the post hoc Bonferroni test was used to identify the significant differences among categories. When main effect and interaction were found, only the interaction effect was reported. The magnitude of treatment effects was calculated using eta squared effect size. In all analyses, significance level of P<0.05 was adopted.
RESULTS
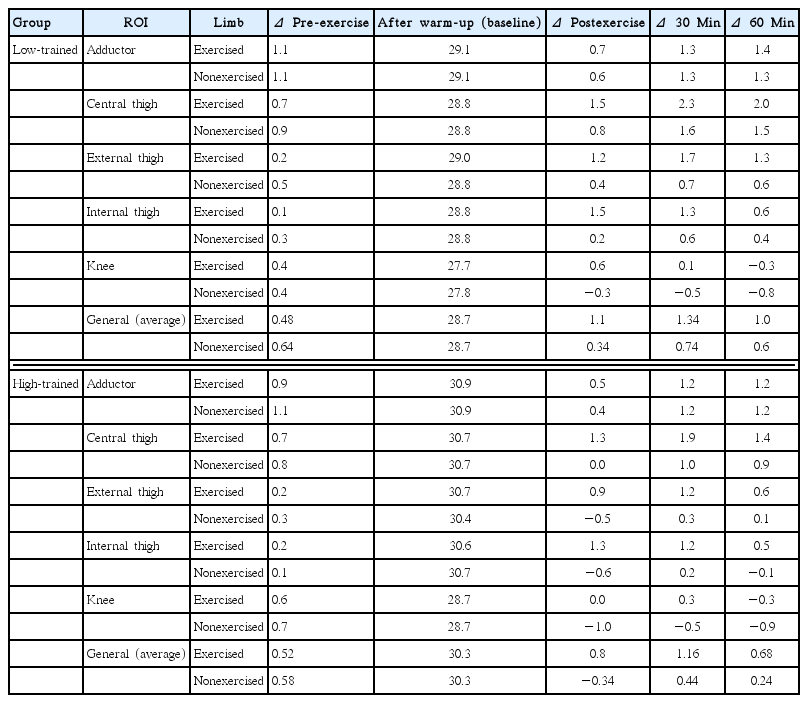
The statistical analysis indicated an interaction effect between the level of training and ROI (F=24.373, P<0.001, η2=0.89) and between the level of training and moment of measurement (F=51.376, P<0.001, η2=0.945); when applied to sphericity correction there was a significant interaction effect between the level of training, ROI and time measurement (F=15.855, P< 0.001, η2=0.514). Table 1 shows the descriptive data (mean± standard deviation) for all the considered ROI for both the exercised (strong) and the nonexercised (weak) legs in the low- and high-trained group. They indicated significant differences between exercised and nonexercised ROIs and between data collection moments.
Table 2 shows the statistical differences obtained between training level by trained leg, ROI, and time of measurement. For exercised leg, in general, the results indicated significant differences on Tsk except for the external thigh (60 min) and knee (after warm-up, for 30 min, for 60 min). For nonexercised leg, significant differences were observed at all measured moments for the adductor and external thigh. in central and internal thigh, we observed differences in all moments of measurement except postexercise.

Summary of temperature (ºC) differences between high- and low-trained group for exercised and nonexercised leg
Table 3 summarizes the evolution of Tsk considering the after warming-up values as the baseline. A general tendency has been estimated by obtaining the average of the considered ROIs. In general, the initial Tsk’s was reduced after warming-up and recovered immediately after exercise, increasing the values 30 min after exercise and remaining higher 60 min after exercise. This tendency was stronger in low-trained group than in high-trained group.
DISCUSSION
Unilateral training can be recommended in different situations of physical training, such as in rehabilitation (Dragert and Zehr, 2011). This study verified the influence of the unilateral strength training on the skin thermal response for both exercised and nonexercised limbs in low- and high-trained subjects. The main results indicated differences immediately after exercise between both exercised and nonexercised limb and low- and high-trained participants in several moments of measurement for all ROI except for the anterior knee. At 30-min postexercise in nonexercised limb differences also were observed in all ROIs except for the anterior knee. The comparison between groups probed that low-trained subjects incremented more the Tsk after exercise in both legs than high-trained. Unilateral training may also influence the contralateral muscles, because the neural signal is unfolding, stimulating commissural interneurons on the spinal cord, which act on the activation of contralateral motoneurons. Also, the neuromuscular system could provide adaptation to the untrained side improving neural drive to the agonist (Carroll et al., 2006; Lee and Carroll, 2007).
Tsk response to the strength exercise
The Tsk behavior has been considered in previous studies, in which agonist muscles obtained maximal Tsk values not immediately after training but during the recovery process (de Andrade Fernandes et al., 2016; Fernández-Cuevas et al., 2014). The increment of Tsk could be due to an increased activation of involved muscles that releases more energy after being exercised (Adamczyk et al., 2014). It is widely known that the increment of temperature during exercise causes disturbances in thermoregulation system of the human body (Formenti et al., 2013) to allow maintaining the temperature homeostasis altered by exercises of different intensity and duration (de Andrade Fernandes et al., 2016; Ferreira et al., 2008). The most relevant thermoregulation system meditated by nervous system tries to become the Tsk symmetrical (Niu et al., 2001). In that way, the body attempts to control the temperature exchange between human body and environment.
In our study, the ROIs corresponding to the muscles involved in exercise had a Tsk increment immediately after exercise. This increase was more specific to the muscles exercised in strength training. This behavior agrees with the results of Fernández-Cuevas et al. (2014) that obtained Tsk increments on agonist muscles after a strength training of upper and lower limbs. However, the effects could be slightly different in our study because the blood flow had to be redistributed only in the lower area. In addition, the expected cross-effect response on the Tsk took place on the homologous agonist’s muscles of the nonexercised leg. A similar physiological response to unilateral training has been obtained previously with unilateral training measured with EMG (Beyer et al., 2016; Pincivero et al., 2006) and, being measured with other methods, the contralateral effect is only produced in the homologous muscles that were doing the activity (Chiesa et al., 2016).
Inner muscle temperature increment after strength training depends on time under tension and speed of contraction of the muscles involved in the activity (Marins et al., 2011). In the same way, Tsk variation registered on the nonexercised leg could also be reflecting the different level of activation in those muscles. To sum up, the energy (heat) produced by the muscle during contraction it is unable to drive out, so it is stored in muscle tissue (Kenny et al., 2008). The restoring of the normal blood flow to the skin after finishing the exercise with blood proceeding from activated muscles increases also the heat in the skin area corresponding to those muscles (Merla et al., 2010).
The causes for the Tsk increment in nonexercised leg are not clear, because it has not been measured Tsk simultaneously with other neural parameters (EMG) or biochemical parameters (levels of creatine-phosphokinase). According to the recent review of contralateral training of Lee et al. (2009), we think that this increment may be linked to the neural mechanisms related to cross effect. In the study of Rattey et al. (2006) the acute fatigue appeared on both exercised leg and on homologous muscles of nonexercised leg seems also to be related to neural factors. Although, it cannot dismiss a muscular factor as a transformation of satellite cells form inactive to active, hypertrophy or change on fiber type interfere in cross effect.
Tsk response to the strength exercise along the time
In the initial rest state, the Tsk should be equal on both sides for humans (Niu et al., 2001). Our data show how nonexercised and exercised legs had symmetrical distributions of Tsk both in high and low-trained subject, on all the considered ROI of the lower limb at pre-exercise, assuming that participants started the training protocols in an optimal rest state. The general decrease of Tsk after warm-up goes in line with the research of Adamczyk et al. (2012). Their study shown that the decrease on quadriceps Tsk is directly related with the power performance measured with a countermovement jump. Other authors provided also information about a fall in the skin temperature in the initial period of exercise (de Andrade Fernandes et al., 2016; Fernández-Cuevas et al., 2014; Marins et al., 2011). The temperature drops immediately after exercise induces a higher relative increase in the subsequent moments of recovery, making more relevant the differences in the moments after training. Hence, in future research it could be important consider the temperature before and after the warm-up to verify the acute effect or behavior of Tsk to the exercise.
Immediately after training, in the exercised leg, the ROIs corresponding to the involved muscles exhibit an increment of temperature, normally significant. It could be inferred during the warm-up it is activated the physiological response of blood flow redistribution in order to provide nutrients to the active muscles facilitated by a vasoconstriction of the skin vessels. The energy generated by the active muscles is not enough to generate a Tsk increase at skin level. However, with a higher volume or intensity of work and a certain time after starting the muscle activity, the heat produced in muscle may reach the skin level being probably transferred by conduction (from hotter inner muscles to colder outer tissues) and mainly by the restauration of the normal blood flow to the skin with warmer blood from the active muscles; so that, the Tsk increases after exercise (Kenny et al., 2008). Further research should be done in order to understand better the dynamics of Tsk due to the different types of exercise.
On the other hand, the Tsk on the nonexercised leg of our subjects remained at the baseline levels immediately after exercise. These results agree with previous studies where, under normal conditions, the temperature tends to be constant (Formenti et al., 2016; Okazaki, 2016). However, 30 min after exercise, were recorded the maximum Tsk values along the study for both legs in the ROIs of the involved muscles. This is a remarkable fact because the nonexercised leg did not perform any activity. They have been not found studies about the contralateral effect on Tsk measured with any alternative method, although the cross-education effects are widely known and it has been demonstrated an increment of strength and an acute contralateral fatigue of nonexercised limb (Lee and Carroll, 2007; Rattey et al., 2006). Our data about the Tsk increase on nonexercised leg 30 min after training could be considered as directly related to the already probed contralateral effect. We will name it as “acute contralateral Tsk effect” (ACTsk), and it could be assumed as half of the increment generated on the exercised leg 30 min after strength training.
Finally, 60 min after training, the Tsk on the muscle areas involved in the performed exercise was maintained or slightly decreased compared to 30 min after exercise and also on the homologous nonexercised muscle, which means that the ACTsk is maintained at least up to an hour after training. The excess postexercise oxygen consumption should be one of the variables responsible for the Tsk increment due to the increased metabolism after exercise (Laforgia et al., 2006). In contrast, the nonexercised muscle areas tend to recover the baseline temperature controlled by the thermoregulatory system.
Differences between high- and low-trained subjects
It is interesting to point out that baseline data (pre-exercise) are more than 1ºC higher in high-trained group (Tables 1, 3). This is probably due to a higher muscle activation and blood flow in high-trained subjects. The higher Tsk values recorded on high-trained indicate that the level of training is a factor of influence to be considered during the interpretation of thermograms after exercise. The thermal behavior differs between high- and low-trained subjects (Formenti et al., 2013). Previous studies have established these differences based on changes of the thermoregulation processes depending on the physical level of the subject (Adamczyk et al., 2016; Boguszewski et al., 2014). Low-trained subjects release the heat worse than high-trained during exercise and maintain the heat longer time during the recovery process (Formenti et al., 2013).
In our study, the low-trained participants showed a higher increment in absolute Tsk values than high-trained after exercise. A probable explanation is that high-trained athletes recruit more synergist muscles to perform the same action reducing the requirements to the agonist muscles. The increment on absolute Tsk could be due to the fact that high-trained delay the Tsk increment and they dissipate the heat better than low-trained (Formenti et al., 2013). In addition, the high-trained athletes performed with higher absolute workload and have more level of performance and better neural drive. These facts could make that high-trained involved more muscles in action than low-trained (Fimland et al., 2009).
This study reinforces the idea of use thermography as sport tool to quantify the training load, providing information about the exercise selection and the effect of training on the body. The acute Tsk crossed effect indicates a training adaptation to unilateral strength training and coaches could utilize the unilateral training as a useful method of strength training; however, they should consider some aspects, as the acute prefatigue generated on nonexercised limb, before performing exercise with the other limb. IRT could be used to assess and monitor the effects of unilateral training during rehabilitation, to prevent loss of strength in the affected or immobilized limb. The differences between the low-trained and the high-trained must be also considered by coaches, since the same exercise involve different activation of the muscle to perform the activity, being highly trained who recruit more synergist muscle probably due to a better coordination or neural communication, and the fact that they mobilize higher absolute load.
To conclude, it can be said that after contralateral training Tsk has a significant increment both on ROIs corresponding to exercised muscles and on the contralateral nonexercised muscles. However, the joints have different behavior exhibiting a detriment on Tsk after training. The peak of Tsk in both exercised and nonexercised leg was produced around 30 min after training. On the nonexercised leg, this increase corresponds to half of exercised leg increment. So that, the Tsk response to contralateral training seems to be related to the cross-effect and set up ACTsk to monolateral strength training. The ACTsk is more accentuated in low-trained than in high-trained subjects. Using IRT as a measurement method, it has been demonstrated an acute Tsk crossed effect in the nonexercised limb as a response to the unilateral strength training.
ACKNOWLEDGMENTS
This study was funded by Technic University of Madrid (UPM).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.