Association between physical activity and self-rated health in pediatric patients with type 1 diabetes mellitus
Article information
Abstract
Patients with type 1 diabetes mellitus (T1DM) tend to experience poor self-rated health. However, few studies have examined the association between physical activity and self-rated health in pediatric patients with T1DM. The purpose of this study was to investigate the association between regular physical activity, regular muscle strength exercise, and self-rated health in pediatric patients with T1DM who lacked diabetes care. The eligible participants for this study were 37 pediatric patients with T1DM aged 9 to 17 years. Physical activity was divided into regular physical activity and regular muscle strength exercise to analyze the relationship with self-rated health using binomial logistic regression analysis. The results showed that self-rated health of pediatric patients with T1DM who did not engage in regular muscle strength exercise was significantly lower than those who did (odds ratio [OR], 0.100; 95% confidence interval [CI], 0.012–0.855; P<0.05). However, the association between regular physical activity and self-rated health was not statistically significant (OR, 0.211; 95% CI, 0.041–1.088; P=0.06). In conclusion, regular muscle strength exercise in pediatric patients with T1DM who lacked diabetes care was effective in contributing to optimal self-rated health. Future research is needed to collect physical activity data using objective assessment methods and to analyze the association between variables applying diverse factors for pediatric patients with T1DM, which might be able to effect on their health.
INTRODUCTION
Interest in the health management of type 1 diabetes mellitus (T1DM) patients has increased in recent years. Research has revealed a 3% rise annually in pediatric T1DM patients internationally (Patterson et al., 2009). Although South Korea is known to have a relatively low incidence rate of T1DM, the incidence rate is rapidly increasing according to recent data (Kim et al., 2016). A number of complicating issues tend to be experienced by those with T1DM diagnoses. For example, T1DM patients may suffer psychologically through a long period of glycemic control during childhood (Beacham and Deatrick, 2015). These psychological issues tend to be complicated further by the pressure of academic work throughout one’s education (Delahanty et al., 2007) and can be compounded by hormone changes during puberty (Court et al., 2009; Helgeson et al., 2010; Lee, 2014). T1DM patients may also have trouble managing their blood glucose (Hood et al., 2014; Selvin et al., 2014). Concern about managing blood glucose can influence high levels of stress, depression, and anxiety among pediatric patients with T1DM (Compas et al., 2012; Delamater et al., 2014). These concerns might affect pediatric patients’ quality of life. For example, researches have indicated that quality of life indices tend to be lower among this population when compared to patients with type 2 diabetes mellitus (Hood et al., 2014; Kalyva et al., 2011).
Among pediatric patients with T1DM, those who lack diabetes care could experience more serious psychological problems and glycemic control issues in a vicious circle (Engum et al., 2005). Therefore, it is important to investigate pediatric patients with T1DM who lack diabetes care. Self-rated health is regarded as one of the most important factors to pediatric patients with T1DM because they have to self-manage their disease for their entire lifetime after being diagnosed. Self-rated health is considered a measure of overall health that considers biological, psychological, social, and functional health levels (Reile et al., 2017). Researchers have identified that poor self-rated health tends to be associated with obesity (Herman et al., 2014) or low-grade inflammation (Warnoff et al., 2016) among this population. Even though a number of studies have verified the positive effect of physical activity on self-rated health (Kwon et al., 2016; Tsai et al., 2010), no previous research has examined this association among pediatric patients with T1DM. Therefore, the purpose of this study was to investigate the association between regular physical activity and self-rated health in pediatric patients with T1DM who lacked diabetes care.
MATERIALS AND METHODS
Participants
The eligible participants of this study were 37 pediatric patients with T1DM that attended a diabetes education program at the endocrinology clinic of “A” University Children’s in South Korea. This study included children and adolescents aged 9–18 years who participated in the program between January 2011 to January 2015. The specific selection criteria were individuals who had: (a) received a T1DM diagnosis at least six months prior, (b) over 7.5% of glycosylated hemoglobin (HbA1c) (American Diabetes Association, 2016), which is a biological value used when monitoring diabetes, and (c) consented to the study.
Data collection and instruments
This retrospective study involving a chart review of patients diagnosed with T1DM was approved by the Institutional Review Board (IRB) of Seoul National University Children’s Hospital (IRB No. H-1606-046-769). Data that were collected included demographic data, body fat (%), and HbA1c levels. Body fat was measured using a body composition analyzer (Inbody3.0, Biospace, Seoul, Korea), and the data on HbA1c obtained from TBA-C16000 (Toshiba, Tokyo, Japan) after fasting for at least 8 hr. The main variables in this study, which were physical activity, muscle strength exercise, and self-rated health of participants, were assessed at the enrollment time in the education program as in the following.
Regular physical activity
Regular physical activity was measured using two questions and responses. The question for moderate physical activity was “Over the past 7 days, how many days did you engage in moderate physical activity (for at least 30 min) that caused a slight increase in your breathing or heart rate (e.g., tennis doubles, volleyball, badminton or table tennis, or any other activity)?”. The vigorous physical activity question was, “over the past 7 days, how many days did you engage in vigorous physical activity such as running, mountain climbing, soccer, basketball or any other activity (for at least 10 min) that caused a substantial increase in your breathing or heart rate?”. Using these two questions, regular physical activity was defined as follows: vigorous physical activity ≥3 days/wk, moderate physical activity ≥5 days/wk. Participants who did not meet the guidelines were classified as ‘no-regular physical activity’ group.
Regular muscle strength exercise
One question was used to measure regular muscle strength exercise. This question read “over the past 7 days, how many days did you engage in muscle strength exercise such as push-ups, sit-ups or weight lifting, etc.?” Using the question, regular muscle strength exercise was coded as a dichotomous variable of regular (≥3 days/wk) or not-regular muscle strength exercise.
Self-rated health
Self-rated health status was measured by one item, originally derived from SF-36 (Bjorner et al., 1998): “In general, how would you rate your current health?” and the response options were (1) very good, (2) good, (3) fair, (4) poor, and (5) very poor. According to the responses, the variable was classified into a dichotomous variable of ‘optimal self-rated health’ and ‘poor self-rated health.’ Scores 1–2 were coded as optimal self-rated health (Kwon et al., 2016).
Statistical analysis
IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) was used to analyze the data. Descriptive statistics were conducted for the demographic and physical activity characteristics of participants. Odds ratio (OR) and 95% confidence Interval (CI) through the logistic regression analysis were calculated to analyze the relationship among regular physical activity, regular muscle strength exercise, and self-rated health. The adjusting variables were age, gender, and duration of T1DM in the analyzing process. Statistical significance was set at 0.05.
RESULTS
Characteristics of participants
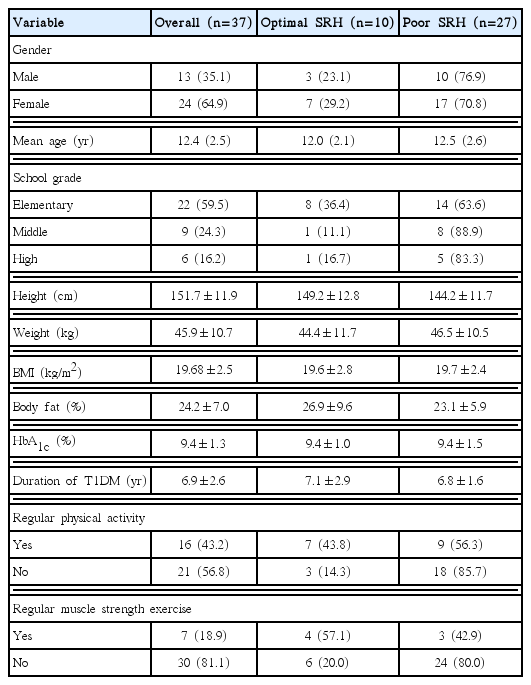
Participant characteristics including descriptive regular physical activity and muscle strength exercise are shown in Table 1. The total number of the participants was 37, which consisted of 13 male (35.1%) and 24 female subjects (64.9%), and their mean age was 12.4±2.5 years. The mean duration of T1DM was 6.9±2.6 years and the mean HbA1c was 9.4%±1.3%. The percentage of optimal self-rated health participants in elementary school-aged was the highest (36.4%, n=8) compared to the participants in middle and high school-aged. Among participants who engaged in regular physical activity, 43.8% reported optimal self-rated health and 56.3% reported poor self-rated health. Among those who did not engage in regular physical activity, 85.7% were considered to have poor self-rated health, whereas just 14.35% had optimal self-rated health. Similarly, 80.0% of those who did not engage in regular muscle strength exercise had poor self-rated health, whereas 20.0% had optimal self-rated health. Lastly, among participants who engaged in regular muscle strength exercise, 57.1% had optimal self-rated health, and 42.9% had poor self-rated health.
Association between physical activity and self-rated health
Table 2 shows the results from the logistic regression models examining the association of self-rated health with participation in regular physical activity and regular muscle strength exercise, respectively. Regarding regular physical activity, no statistically significant interactions were found between regular physical activity and self-rated health after adjustment for age, gender, and duration of T1DM (P=0.063). The participants who did not have regular physical activity were less likely to report self-rated health (OR, 0.211; 95% CI, 0.041–1.088), whereas self-rated health was significantly associated with regular muscle strength exercise (P<0.05). The participants who did not perform muscle strength exercise more than 3 days a week were less likely to express self-rated health (OR, 0.100; 95% CI, 0.012–0.885).
DISCUSSION
Approximately 20%–40% of people with diabetes in South Korea appropriately manage their blood glucose (Choi et al., 2009). However, only 13%–15% of pediatric patients with T1DM are in good management (Livingstone et al., 2012). Therefore, an interest in the health management for pediatric patients with T1DM is a pressing concern. According to the results of this study, pediatric patients with T1DM who did not perform regular muscle strength exercise had lower self-rated health than those who did. And those who did not engage in regular physical activity showed lower self-rated health than those with regular physical activity engagement. This result was synonymous with the many preceding studies that showed that regular physical activity and regular muscle strength exercise positively affected the development of self-rated health (Galán et al., 2013; Hansen et al., 2013). Although difficult to compare because of a lack of concrete physical activity guidelines for maintaining health in pediatric patients with T1DM, the American Diabetes Association recommended that pediatric patients with diabetes perform moderate and vigorous physical activity over 60 min every day and muscle strength exercise more than 3 days a week (Copeland et al., 2013). As the results mentioned above, the association between self-rated health and regular physical activity was not statistically significant. But, there was a tendency toward lower self-rated health in pediatric patients with T1DM engaging in no regular physical activity compared to those who did. There might be potential factors that impact on the association between self-rated health and regular physical activity, which may provide some difficulties when offering different and appropriate forms of regular exercise management for pediatric patients with T1DM (Riddell et al., 2017). Additionally, the participants’ recognition of the amount and intensity of physical activity might not be sufficient to report it.
The present study showed that moderate physical activity of more than 150 min per week or vigorous physical activity of more than 60 min per week tended to positively effect on self-rated health in pediatric patient with a lack of diabetes care. However, the amount of physical activity they engaged in did not reach the recommended amount of physical activity per week by American Diabetic Association. The results also showed that pediatric patients who lacked diabetes care and engaged in muscle strength exercise more than 3 days per week had higher self-rated health than those who did not meet these exercise thresholds. This result is aligned with Copeland et al. (2013), who presented the muscle strength exercise guidelines for this population. A having higher self-rated health in pediatric patients who lack diabetes care who performed regular physical activity and muscle strength exercise than those who did less exercise could be explained. First, gaining confidence may come from an improvement in body shape and physical fitness through participating in regular physical activity or muscle strength exercise (Nguyen et al., 2015; Porter et al., 2017). Second, children and adolescents with sufficient physical activity are more likely to report more positive health conditions because they tend to have low levels of negative mental health (Gunnell et al., 2016). Furthermore, this was regarded to influence the self-rated health of pediatric patients with T1DM positively by increasing happiness (Min et al., 2017) which came from social interaction effects in performing physical activity with peers (Price and Weiss, 2013).
Several limitations may be noted for this study. For example, the small sample size, which was due to only targeting patients in a diabetes education program with intensified treatment at a specific location, limited potential data analyses. Thus, a careful interpretation is necessary to generalize the results. A further limitation is the inability to establish a causal connection between the independent and dependent variable based on analyzing cross-sectional data. The present study did not adjust to use other variables to explore effects on the self-rated health. In the future research, an advanced data analysis is needed to consider gender or other demographic variables as well as colleting the data to use objective instruments, such as accelerometers and pedometers.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2015S1A5A07042494).