Effects of spinal stabilization exercise with real-time ultrasound imaging biofeedback in individuals with chronic nonspecific low back pain: a pilot study
Article information
Abstract
The use of real-time ultrasound imaging (RUSI) as biofeedback to enhance the performance of spinal stabilization exercise and recovery from low back pain has been a recent trend in musculoskeletal rehabilitation. The aim of this pilot study was to evaluate whether it would be feasible to conduct a randomized controlled trial investigating the effects of spinal stabilization exercise with RUSI biofeedback in individuals with chronic nonspecific low back pain. This was a single-group pretest-posttest quasi-experimental study. Ten consecutive patients with chronic nonspecific low back pain met the study criteria. They received spinal stabilization exercise with the RUSI biofeedback focusing on lumbar multifidus muscle activation. The intervention was provided twice weekly for 6 weeks. Outcome measures were lumbar multifidus muscle cross-sectional area, pain, disability and quality of life assessed at baseline and after intervention. A paired t-test was applied and effect size (Cohen d) was computed. The recruitment and retention rates were 75% and 83% respectively. No adverse events were reported during the study. Compared with the baseline, the participants demonstrated statistically significant improvement in lumbar multifidus muscle cross-sectional area (P<0.05, d=1.03), pain (P<0.001, d=2.56) and disability (P<0.05, d=1.43) with large effect size after the intervention. However, no statistically significant differences were observed for physical and mental health (P>0.05) after the intervention. It was concluded that spinal stabilization exercise with RUSI biofeedback is effective in improving lumbar multifidus muscle cross-sectional area, pain and disability in individuals with chronic nonspecific low back pain. The results demonstrated the feasibility of conducting a future, larger-scale powered randomized controlled trial to confirm these preliminary findings.
INTRODUCTION
Low back pain (LBP) has been considered to be one of the most painful health problems worldwide and recorded to be the most commonly managed condition in healthcare settings (Hartvigsen et al., 2018; Kovacs et al., 2012). It is defined as pain, muscle tension, or stiffness localized below the 12th costal margin and above the inferior gluteal folds, with or without leg pain (Chou, 2010). LBP is a major public health issue as it adversely affects the patient’s well-being and quality of life, and contributes largely to the burden of disease globally (Dagenais et al., 2008).
The burden of LBP is enormous in terms of economic, health and societal costs. The direct and indirect cost of LBP in the US has been reported between $84.1 billion and $624.8 billion, with lost work productivity resulting in indirect costs of $7.4 billion to $28 billion (Dagenais et al., 2008). The incremental expenditures due to LBP were estimated around $26 billion (Luo et al., 2004). Moreover, the burden of LBP is projected to upsurge in the coming decades especially in low-income and middle-income countries mainly due to population growth and aging (Hartvigsen et al., 2018).
According to previous reviews, the prevalence of LBP in Africa is rising and comparable to that of western nations (Louw et al., 2007; Morris et al., 2018). In a review conducted by Morris et al. (2018), the annual LBP prevalence among Africans was found to be 57% which is considerably higher than the 38.5% estimated globally (Hoy et al., 2012). In another review, it was found that workers (48%) are the most common population group presenting with LBP while scholars comprised 15% of the population (Louw et al., 2007). In Nigeria, the annual prevalence of LBP has been reported ranging between 40% and 74%, which affects mostly the rural income communities (Omokhodion, 2002; Tella et al., 2013).
Although LBP is often considered as a complex or multifactorial disorder with many potential causative factors, in majority of cases (90%), the cause of the pain is unknown which is commonly referred to as nonspecific LBP (Balagué et al., 2012). However, there are different interpretations of the underlying mechanisms of pain even when specified radiological diagnoses are established (Hildebrandt et al., 2017). One mechanism that has been linked with LBP is the altered stability and motor control of specific muscles of the trunk. Morphological studies show that lower cross-sectional area (CSA) (Danneels et al., 2000; Hides et al., 2008; Kamaz et al., 2007) and a greater percentage of intramuscular fat (Alaranta et al., 1993; Mengiardi et al., 2006) in the lumbar multifidus muscle is evident in individuals with chronic LBP. Moreover, there is evidence to suggest that training leading to activation and strengthening of the lumbar multifidus muscle may alleviate symptoms of LBP (Goldby et al., 2006; Hides et al., 2008; Ibrahim et al., 2018). Thus, rehabilitating this musculature is considered essential for individuals with chronic low back disorders.
The use of real-time ultrasound imaging (RUSI) as biofeedback to enhance the performance of spinal stabilization exercise and recovery from LBP has been a recent trend in musculoskeletal rehabilitation. RUSI has been used successfully to evaluate the thickness of deep trunk muscles (Akbari et al., 2008; Ehsani et al., 2019; Shamsi et al., 2016) and to provide visual feedback of these muscles’ activation in both healthy and symptomatic individuals (Henry and Teyhen, 2007; Henry and Westervelt, 2005; Lee et al., 2018; Van et al., 2006). While specifically the use of RUSI has been shown to improve preferential activation of the lumbar multifidus muscle in individuals with LBP (Hides et al., 1996; Hides et al., 2012), however, the effects of this treatment approach in individuals with chronic nonspecific LBP (CNLBP) appeared not to have been widely reported. However, prior to conducting a larger-scale randomized controlled trial (RCT) to test the efficacy of this treatment approach, it is important to carry out a pilot study to evaluate the feasibility for such a larger trial so that resources and efforts are not wasted (Thabane et al., 2010).
Therefore, the aim of this pilot study was to evaluate whether it would be feasible to conduct a RCT investigating the effects of spinal stabilization exercise with RUSI biofeedback in individuals with CNLBP.
MATERIALS AND METHODS
Study design
This pilot study adopted a single-group pretest-posttest quasi-experimental design and was conducted between July and October 2018 at the out-patient unit of physiotherapy department, National Orthopedic Hospital, Dala, Kano State, Northwestern Nigeria.
Ethical consideration
Ethical clearance was sought and obtained from the Research and Ethical Committee of National Orthopedic Hospital, Dala, Kano State (Ref: NOHD/RET/ETHIC/60) before the commencement of the study.
Participants and flow
The participants were consecutive patients with chronic LBP referred for physiotherapy by consultant orthopedic surgeons from the general out-patient department of National Orthopedic Hospital, Dala, Kano State. The participants were eligible for the study if they were both male and female between the age of 18 and 60 years, had primary complaint of nonspecific LBP of at least 3-month duration with and without pain radiating to one or both lower limbs, and able to read and understand English language and or Hausa language. Participants were excluded if they had a history of spine surgery, pregnancy and serious spinal pathology such as fracture, infection and metastases. Other exclusion criteria included a history of neurosis, depressive symptomatology and other LBP yellow flag factors as all these factors could potentially skew the data.
The eligibility of the participants was ensured by the primary investigator (RS) through history taking and physical examination. All participants were made to sign an informed consent after detailed information of the study was provided. It was also made known to them that their participation in the study was free and can discontinue from the study if so wish. Participants were also informed about the importance of adherence to protocol and prescribed home program when the intervention started.
Baseline assessments
Demographic data consisting of age, gender, body mass index (BMI), duration of back pain, and occupational status were collected using a researcher designed form. Clinical outcome assessments involved measurement of lumbar multifidus muscle CSA with a D3 ultrasonic diagnostic imaging system (primary outcome) and evaluation of pain, functional disability and quality of life using self-report measures.
Sample size estimation
A formal sample size calculation was not conducted for this study since it was a pilot study. However, we thought that recruiting 10–12 participants would be adequate to give an insight into the study feasibility.
Outcome assessments
Recruitment rate
This was expressed as the percentage of eligible participants that consented to participation (Ibrahim et al., 2018).
Retention rate
This was expressed as the percentage of participants who completed the intervention without loss to follow-up. An acceptable retention rate was set at least half (50%) of the participants completing the interventions (Ibrahim et al., 2018).
Adverse events
All participants were informed prior to intervention to contact the research coordinator via phone or during follow-up visits in case they experience any unexpected serious adverse event such as exacerbating back pain, excessive fatigue, light-headedness. And shortness of breath or dizziness during the study.
Lumbar multifidus muscle CSA
The imaging of the lumbar multifidus muscle CSA was assessed using a D3 ultrasonic diagnostic imaging system with 5-MHz coplanar transducer. The procedure for the measurement is identical to that described by Sokunbi et al. (2008). Participants were asked to assume prone lying position with proper pillow support and the neck turned to the participants’ preferred side. The lumbar spine was palpated cranially from the line joining the superior border of the iliac crest (L4/L5) to locate the position of L5 spinous process. It is a deep small blunted bony point lying at the center of the lumbosacral depression. L5/S1 position was checked and confirmed against the lumbosacral depression as seen on the ultrasound image. The ultrasound imaging transducer head with a coupling medium was then placed at the L5/S1 level and moved laterally and angled in a cephalocaudal direction to obtain a clear visualization of the zygapophyseal joints, muscle bulk, thoracolumbar fascia, and echogenic lamina of L5 spinous process. The clearest image of lumbar multifidus muscle was captured and the CSA was determined as stipulated in the manual of the D3 ultrasonic diagnostic imaging system version.
Pain intensity
The levels of the pain intensity of the participants were assessed by administering the Hausa Numerical Pain Rating Scale (NPRS; 0–10 cm) (Ibrahim et al., 2020a), with 0 representing “no pain” and 10 representing “worst imaginable pain.” The participants were asked to mark the number that best reflects their current pain at rest. The Hausa NPRS has been shown to be a reliable, valid and responsive measure of pain intensity among patients with chronic LBP (Ibrahim et al., 2020a).
Functional disability
This was assessed by administering the Roland Morris Disability Questionnaire (RMDQ). It is a 24-item questionnaire, and the scores range from 0 (no disability) to 24 (maximum disability). The participants were asked to choose from the 24 items as it suitable for their current disability level. The RMDQ score is calculated by adding up the number of items checked. The RMDQ has good psychometric properties in terms of reliability and validity (Chiarotto et al., 2016; Roland and Fairbank, 2000). Prior to data collection, the questionnaire was translated into Hausa using the recommended guidelines for translation and cross-cultural adaptation of self-reported measures (Beaton et al., 2000).
Quality of life
The quality of life of the participants was assessed by administering the Hausa short-form health survey (SF-12) questionnaire (Ibrahim et al., 2020b). It is a shorter version of the SF-36 which comprises physical component scores (PCS-12) and mental component scores (MCS-12). To compute the PCS-12 and MCS-12, a web-based scoring tool (www.orthotoolkit.com/sf-12/) was used. Higher scores reflect better health status. The Hausa SF-12 was found to have adequate psychometric properties in terms of reliability, construct validity and factorial invariance among patients with chronic LBP (Ibrahim et al., 2020b). All clinical outcomes (lumbar multifidus muscle CSA, pain intensity, functional disability and quality of life) were assessed at baseline and after treatment.
Interventions
All the interventions in this study (i.e., stabilization exercise with RUSI biofeedback) were provided by the primary researcher and a research assistant (AI) who was a consultant radiologist with over 10 years of experience in diagnostic ultrasound imaging. Participants were instructed on how to activate and contract the lumbar multifidus muscle in synergy with other muscles of core stabilization using bracing methods. Participants were asked to assume prone lying with proper pillow support and the neck turned to the participants’ preferred side as described under the lumbar multifidus muscle CSA measurement. While in this position, the ultrasound transducer head was placed at the L5/S1 level and the participants were then instructed to contract the core stability muscles as previously thought. They were also instructed to focus on the monitor to see the changes in the thickness of lumbar multifidus muscle CSA as they undergo the contraction and put in their best effort to increase the thickness with successive contractions. Ten sets of contractions, holding each contraction for 10 sec were carried out with a period of 2-min rest in between contractions. The entire exercise period lasted for 30 min. Treatment was carried out twice a week for 6 weeks.
Data analysis
All statistical analyses were conducted using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) with 0.05 set as level of significance. Normality test for the dependent variables was verified using the Shapiro–Wilk test. Descriptive statistics of mean, standard deviation, frequencies and percentages were used to summarize the data. Inferential statistics using paired t-test (normally distributed data) was applied to analyze the differences between pre- and postintervention in lumbar multifidus muscle CSA, pain intensity, functional disability and quality of life mean scores. Effect size was calculated using the formula d=t/√N, where t is t-score and N is the total sample size. Effect size values were considered according to Cohen d as either trivial (<0.2), small (≥0.2 and <0.5), moderate (≥0.5 and <0.8), or large (≥0.8).
RESULTS
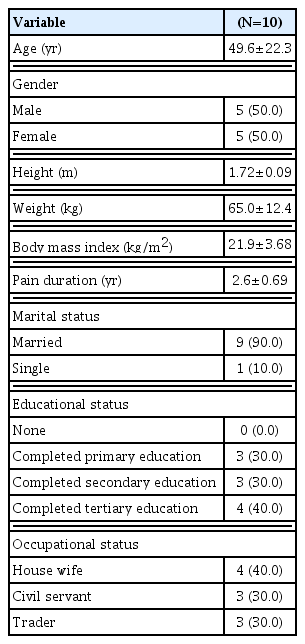
Sixteen participants were assessed for the study eligibility of which 12 fulfilled the inclusion criteria, yielding a recruitment rate of 75% (12 of 16). Two of the excluded participants were due to the inability to fulfill our age criteria (i.e., >60 years) and the other two had a history of back surgery. Of the 12 consented participants, only 10 (five males and five females) completed the intervention representing a retention rate of 83% (10 of 12). The reasons for the dropout were serious illness and traveling. The participants reported no adverse events during the study. The socio-demographic characteristics of the participants are presented in Table 1. The participants’ mean age and BMI were 49.6±22.3 years and 21.9±3.68 kg/m2 respectively.
Table 2 shows changes in clinical outcomes after the intervention among the participants. Results of the paired t-test revealed statistically significant differences in lumbar multifidus muscle CSA (P=0.010, d=1.03), pain intensity (P=0.000, d=2.56), and functional disability (P=0.001, d=1.43) with large effect size after the intervention. However, no statistically significant differences were observed for physical and mental health (P>0.05) after the intervention (Table 2).
DISCUSSION
This pilot study assessed the feasibility of conducting a future larger-scale, powered RCT to examine the efficacy of spinal stabilization exercise with RUSI biofeedback in individuals with CNLBP. The results of the study suggested the feasibility in terms of recruitment rate, retention rate, safety and potential effects of treatment on lumbar multifidus muscle CSA, pain intensity and functional disability.
The feasibility of conducting a larger-scale, powered RCT was demonstrated by the excellent recruitment and retention rates as well as safety of the intervention. Even though we did not recruit a large number of participants in the current study, the recruitment period was short and only four participants out of the 16 assessed were excluded. One of the reasons for the exclusion was due to overage (>65 years). This criterion, however, would be relaxed while recruiting participants for the large RCT to have more representation. Interestingly, there was an equal distribution of the participants with regard to gender signifying that both males and females were equally affected and gender bias was eliminated.
Regarding lumbar multifidus muscle CSA, it has been well documented that this muscle is atrophying in individuals suffering from LBP with or without leg pain (Danneels et al., 2000; Hides et al., 2008; Kader et al., 2000; Kamaz et al., 2007). This was also anticipated among the participated individuals in the current study as they were all sufferers of chronic LBP. The stabilization exercise with RUSI biofeedback employed proved to be valuable in activating their lumbar multifidus muscle evidenced by the improvement in the CSA with large effect size after completion of the study despite the smaller sessions of treatment. In support of this finding, the use of RUSI as a biofeedback tool has been reported by several authors to enhance preferential activation of the lumbar multifidus muscle in individuals with (Hides et al., 1996; Hides et al., 2008; Maraschin et al., 2014) or without (Van et al., 2006) LBP. According to the above authors, preferential activation of the lumbar multifidus muscle was significantly associated with its recovery, performance, and retention in the ability to activate the muscle as well as a limited number of treatment sessions. The focus of stabilization exercise is to improve the activation pattern of deep trunk muscles and restore functional posture and movement control, which in turn may help to relieve lumbar pain and instability (Goldby et al., 2006; Kavcic et al., 2004; Kim and Kim, 2018). Thus, the participants in our study were placed on this training with the aim of restoring the activation pattern of the deep stabilizing muscles of the spine to achieve these desired therapeutic effects.
Promising results were also demonstrated in this study in terms of improved pain intensity and functional disability. The effect sizes were also large, thus suggesting that the magnitude of the changes observed was clinically relevant. This indicates the ability of stabilization exercise as also reported by many authors (Akodu and Akindutire, 2018; Bhadauria and Gurudut, 2017; Ibrahim et al., 2018; Ojoawo et al., 2017) to improve symptoms of pain and disability in patients with CNLBP in the short-term. However, contrary to the findings of previous studies (Golby et al., 2006; Shaughnessy and Caulfield, 2004), we found no significant differences in the physical and mental health outcomes after the intervention which might be partly explained by the shorter treatment sessions (12 treatment sessions) provided. Most studies reporting significant improvement in quality of life in patients with CNLBP have longer treatment sessions (≥16 treatment sessions) (Goldby et al., 2006; Macedo et al., 2012; Shaughnessy and Caulfield, 2004).
One obvious limitation of this study is the quasi-experimental design without a control group hence, limiting the study’s ability to conclude a causal association between the intervention and outcomes. Another obvious limitation is the small sample size and as such the results should be interpreted with caution. Furthermore, outcomes were assessed in the short-term. However, it is important to note that this is a pilot study conducted to evaluate the feasibility of a future, large powered RCT so that resources and efforts are not wasted. Using the results of the present study, refinement of the larger RCT will be done especially regarding the use of comparative and control groups, inclusion criteria (e.g., broader age range), the number of sessions required to induce changes in quality of life, longer follow-ups and other procedural aspects of the study. The results will also be considered in calculating the sample size needed for the future, large RCT.
In conclusion, this pilot study suggest that it is feasible with minor adjustment to conduct a larger scale, powered RCT to examine the efficacy of spinal stabilization exercise with RUSI biofeedback in individuals with CNLBP.
ACKNOWLEDGMENTS
The authors would like to thank all the participants for their participation in the study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.