The effect of an Olympic distance triathlon on the respiratory muscle strength and endurance in triathletes
Article information
Abstract
High-intensity exercise, marathons, and long distances triathlons have been shown to induce the fatigue of respiratory muscles (RMs). Never-theless, fatigue and the recovery period have not been studied in re-sponse of an Olympic distance triathlon (1.5-km swim, 40-km bike, 10-km run: short-distance triathlon). The aim of this study was to evaluate the RM fatigue induced by an Olympic distance triathlon. Nine male triath-letes (24±1.1 years) underwent spirometric testing and the assessment of RM performance. Respiratory function tests were conducted in sit-ting position. Spirometric parameters, maximal inspiratory and expirato-ry pressures, and RM endurance assessed by measuring the time limit were evaluated before (pre-T), after (post-T), and the day following the triathlon (post-T-24 hr). Residual volume increased: pre-T vs. post-T (P<0.002), maximal inspiratory pressure significantly decreased from 127.4±17.2 (pre-T) to 121.6±18.5 cmH2O (post-T) (P<0.001) and returned to the pre-T value 24 hr after the race (125.0±18.6). RM endurance sig-nificantly decreased from 4:51±0:8 (pre-T) to 3:13±0:7 min (post-T, P< 0.001) and then remained decreased for 24 hr after the race from 4:51± 0:8 (pre-T) to 3:39±0:4 min 24 hr after (P<0.002). Both, strength and en-durance of inspiratory muscles decrease after Olympic distance triath-lon. Furthermore, the impaired of inspiratory muscle endurance 24 hr after the race suggested a slow recovery and persistence of inspiratory muscle fatigue.
INTRODUCTION
Respiratory muscle (RM) performance, defined as the capacity of RM to achieve contractile functions, depends on strength and endurance. Inspiratory strength is assessed from the twitch transdiaphragmatic pressures (Walker et al., 2011; Wüthrich et al., 2013). Expiratory strength is assessed from static mouth pressure during maximal expiratory effort (PEmax) (Black and Hyatt, 1969). RM endurance is assessed from maximal voluntary ventilation (MVV), whereas inspiratory and expiratory muscle endurance are assessed from inspiratory (75% PImax) and expiratory (80% PEmax) loaded breathing, respectively, and the measurement of the endurance time limit (Tlim), i.e., the length of time before task failure (Perret et al., 1999).
Decreases in transdiaphragmatic pressure (Walker et al., 2011), maximal inspiratory and expiratory pressures (PImax and PEmax) have been well documented after laboratory exercise (Katayama et al., 2012; Verges et al., 2007) and marathon races (Chevrolet et al., 1993; Ross et al., 2008; Tiller et al., 2019). Similarly, MVV and Tlim for inspiratory muscles were decreased after long-term exhaustive exercise (Bender and Martin, 1985; Boussana et al., 2003) and both 24 hr (Warren et al., 1989) and 3 days (Ker and Schultz, 1996) postultramarathon.
Although RM performance has been studied during a long-distance triathlon (3.8-km swim, 180-km cycle, and 42.195-km run) (Hill et al., 1991), within 10 min of race completion and on the morning after, it has never been studied after an Olympic triathlon at very high-intensity levels. Recently, Smith et al. (2014) and previously, Johnson et al. (1993) reported that exercise at 85% maximal oxygen uptake (VO2max) induced fatigue and decreased blood flow in inactive limb. Indeed, in the two above-cited studies, the authors pointed to the intensity (>85% VO2max) of the exercise more than the duration as the cause of fatigue on RM. The Olympic distance triathlon is the standard race for the Olympics and World Championships (1.5-km swim, 40-km cycle, 10-km run), it is a heavy-intensity, sustained exercise which could impinge on the capacity of RM. However, the impact and recovery period of a such effort on RM performance in real competition received little attention. RM performance has been shown to be impaired after cycle-run successions in laboratory conditions (Boussana et al., 2001), but never investigated after an Olympic distance triathlon.
As the decreases in RM endurance recover less rapidly than the decreases in RM strength (Ker and Schultz, 1996; Ross et al., 2008), we hypothesised that inspiratory muscle endurance impairment following an Olympic distance triathlon would not be completely reversed 24 hr after the race. To verify this hypothesis, we compared the performance of inspiratory muscle before an Olympic triathlon, after the race, and during the recovery period in highly competitive male triathletes.
MATERIALS AND METHODS
Participants
Nine highly competitive male triathletes participated in this experiment. They were 24±1.1 years old, weighed 69.0±1.3 kg, and were 173.1±1.2 cm tall. Before participating in this study, participants performed spirometry test and all had normal spirometric parameters: forced expiratory volume in 1 sec (FEV1), forced vital capacity (FVC) >80%, and FEV1/FVC ratio >75% compared to American Thoracic Society (1986) values. Participants were members of the university athletics team of the University. They trained regularly 16±2 hr per week and had no respiratory abnormalities or cardiovascular disease. They competed at a national or international level (Table 1). Before participating in this research, they all gave written consent approved by the local ethics committee. The study was in accordance with the Helsinki Declaration.
Procedure
The study followed a repeated-measures experimental design. The triathletes trained in maximal respiratory manoeuvres as well as the cycloergometer test prior to testing. They performed an incremental cycle test before entering a three-phase protocol that took place prior to the triathlon, after the race, and 24 hr after the race. An electromagnetic cycle ergometer (Monark 864, Monark-Crescent AB, Varburg, Sweden) was used to perform the incremental test. After a 3-min warm-up at 30 W, the power was then increased by 30 W every minute until the subject reached volitional fatigue (Galy et al., 2013) and maximal oxygen uptake (VO2max) and ventilatory threshold (Thvent) was assessed according to conventional criteria (Beaver et al., 1986).
The three phases of the protocol were performed on different days, but always in the afternoon. Phases 1, 2, and 3 each consisted of a spirometric test and measurements of PEmax, PImax, and RM endurance (Tlim). Phase 1 was conducted 2 to 5 days before the triathlon. The participants were required to avoid competition for the week before the triathlon, but daily training was authorized except on the trial day. Phase 2 was conducted 2:00±0:30 hr after the race. The postrace spirometric test, RM strength, and endurance were performed as rapidly as possible, because that the distance between the race location and the hospital center was 20 km. Phase 3 was conducted 24:00±3:00 hr after phase 2. All the tests were made in laboratory, in ambient room conditions of 755 mmHg, 21°C–22°C and 50% humidity. The Olympic distance triathlon was held at the Grande Motte. The water had a temperature of 14°C and the ambient temperature was 18°C.
Evaluation of spirometry test and RM strength
FEV1, FVC, residual volume (RV), functional residual capacity (FRC), total lung capacity, and maximal expiratory flows were measured by spirometry (Pulmonet III, Sensor Medics, Anaheim, CA, USA) according to standard techniques and procedures. Lung volumes and expiratory flows were compared with reference values (American Thoracic Society, 1986) to ensure that the triathletes had values within the normal range. RM strength i.e., maximal expiratory and inspiratory pressures tests were assessed in the sitting position using the technique of Black and Hyatt (1969). According to this method, triathletes perform tests until three reproducible measurements were obtained and the best value for each athlete was reported (Hayot et al., 2000).
RM endurance
RM endurance was measured by assessing the Tlim with a standardized method using controlled breathing developed by Matecki et al. (2001) and used in previous studies (Boussana et al., 2003; Koechlin et al., 2005). Briefly, Tlim represents the maximal time a subject can breathe against a predetermined inspiratory submaximal load (Roussos et al., 1979). During the experimental run, a threshold valve was connected to the inspiratory side of the respiratory system, as previously described (Roussos et al., 1979). The measurement of the RM endurance was performed using a threshold inspiratory muscle trainer valve (Threshold Inspiratory Muscle Trainer; Healthscan Products Inc., Cedar Grove, NJ, USA) that allows the imposition of a controllable pressure sensor (Johnson et al., 1996; Zocchi et al., 1993). The time limit of RM is performed at the functional residual capacity against a load of 75% PImax placed on the inspiratory orifice and imposed on the athlete with each inspiration (Ker and Schultz, 1996). The test is stopped when the subject no longer maintains the target tidal volume for three consecutive breaths or when he releases the mouthpiece. At that moment, it reaches the limit time of respiratory endurance.
Statistical analysis
The pre-T, post-T, and post-T-24 hr spirometric parameters and RM performance variables were compared using repeated-measures analysis of variance (ANOVA). Scheffé post hoc test was carried out when significant results were obtained with ANOVA. Statistical significance was set at P<0.05. Results are expressed as means± standard deviations. Statistical analyses were performed with SYSTAT software.
RESULTS
The triathletes’ general characteristics are presented in Table 1: age, weight, height, training regimens, triathlon time performances (2 hr 08±0.03 min), VO2, heart rate values at exhaustion, and Thvent of incremental exercise.
Spirometry and RM strength
The comparison between values for FEV1, FVC, FEV1/FVC, forced expiratory flow (FEF25%–75%) in the middle half and forced expiratory flow rate at 50% (FEF50%) before, after and 24 hr after the triathlon remained mostly unchanged. In contrast, RV and FRC increased significantly after compared to before the triathlon (P<0.002) (Table 2). There was no significant change in PEmax before (117.6± 12.8 cmH2O), after (115.8±14.1 cmH2O) and 24 hr after the triathlon (116.0±14.0 cm H2O) (Fig. 1). PImax showed a significant 4.5% decrease from before to after (127.4±17.2 cmH2O and 121.6±18.5 cmH2O, respectively, P<0.001) but had returned to the baseline value at 24 hr after the triathlon (125.8±18.6 cmH2O) (P>0.23) (Fig. 2).

Mean spirometric parameter values measured before (pre-T), after (post-T), and 24 hr after triathlon (post-T-24 hr)
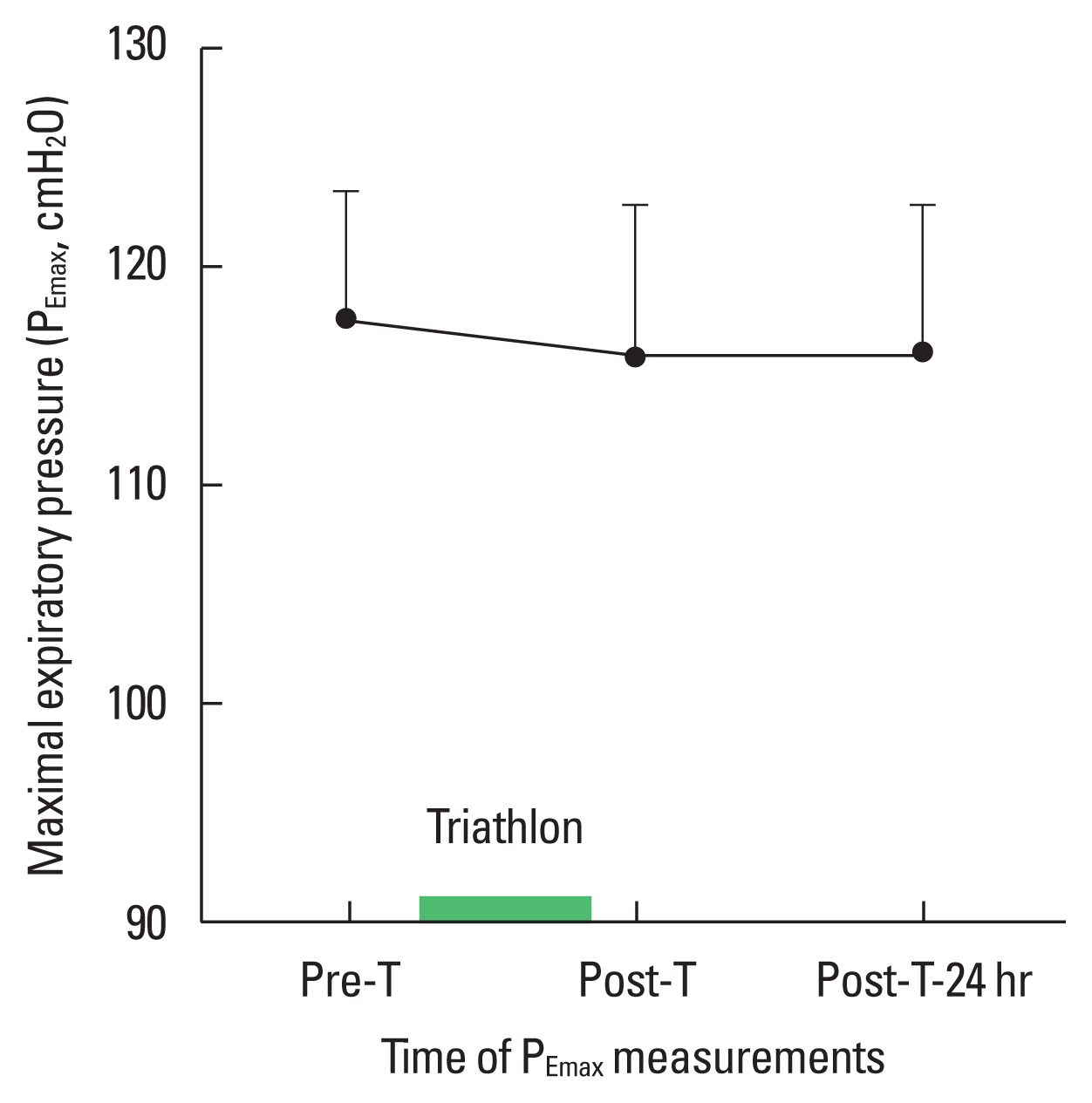
Maximal expiratory pressure (PEmax) pre-T versus post-T and post-T-24 hr. No significant difference between the two trials.
RM endurance
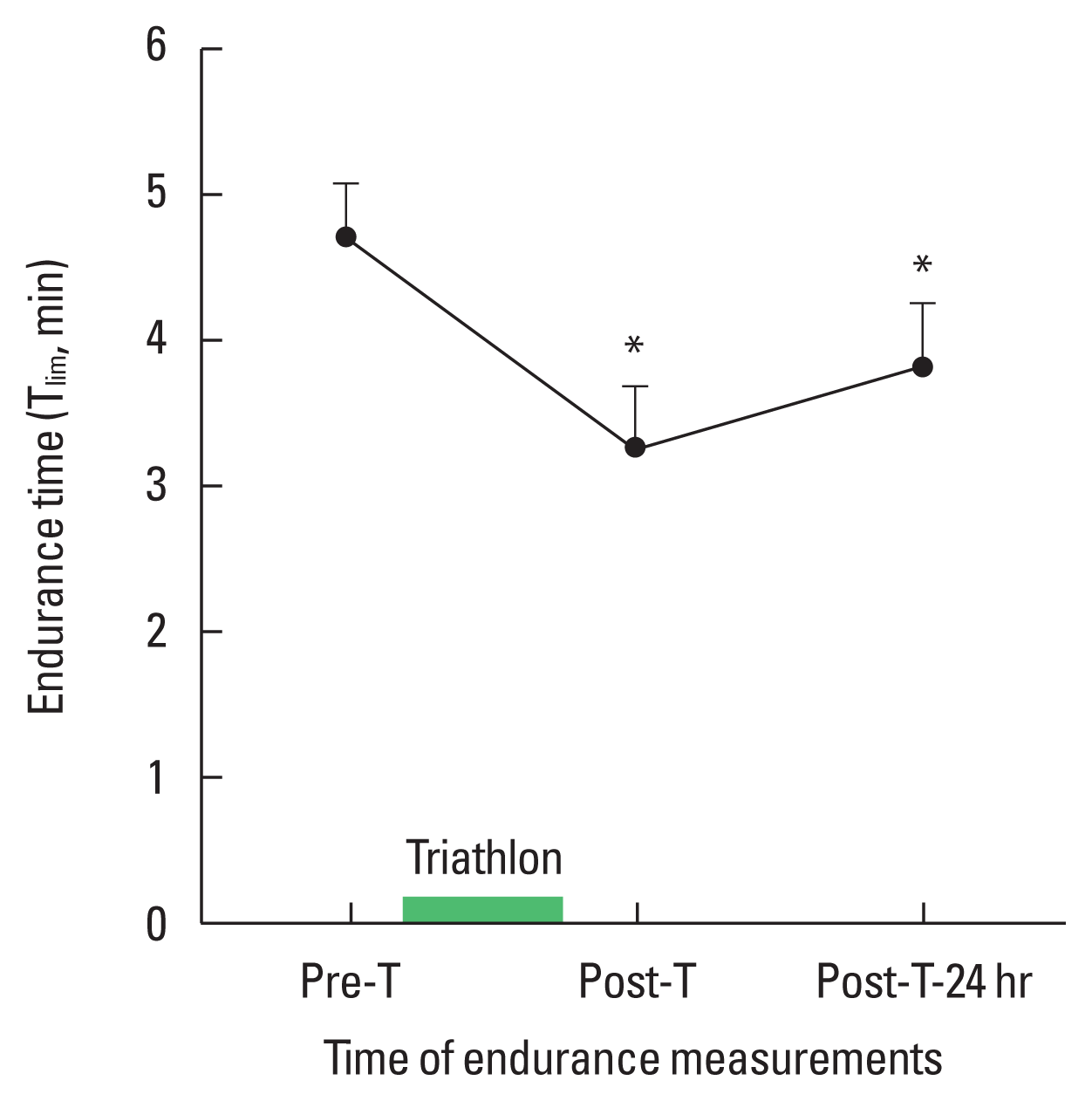
RM endurance (Tlim) showed a 30.5% decrease from pre-T (4:51±0:8 min) to post-T (3:13±0:7 min) (P<0.001), and a 24.8% decrease from pre-T to post-T-24 hr (3:39±0:4 min) (P< 0.002) (Fig. 3).
DISCUSSION
The observation of the main results allowed us to conclude that an Olympic distance triathlon induced a 30.5% decrease in inspiratory muscle endurance in triathletes after the race and that 24 hr later it remained decreased by 24.7% (Fig. 3). These changes suggest that the inspiratory requirements during an Olympic distance triathlon impaired inspiratory muscle endurance.
The 4.5% decrease in maximal inspiratory pressure (PImax: Fig. 2) noted after the Olympic distance triathlon was in line with the 13% decrease in maximal inspiratory pressure reported after strenuous endurance exercise (85% VO2max) (Smith et al., 2014). To explain these results, these authors assumed that inspiratory muscle fatigue was preferentially due to the high exercise intensity (>85% VO2max).
The reduced in maximal inspiratory pressure after the Olympic distance triathlon was consistent with previous findings after an endurance triathlon (Hill et al., 1991), a marathon race (Chevrolet et al., 1993), and laboratory exercise (Katayama et al., 2012). As it is unlikely that the exercise intensity in these studies was continuously above 85% VO2max, one might suggest that a decrease in inspiratory muscle strength also occurred after long duration and submaximal intensity exercise. Inspiratory—or diaphragmatic—work as evaluated by the duration-intensity exercise product could thus be implicated in the decrease in inspiratory muscle performance. The decrease in transdiaphragmatic pressure and inspiratory muscle strength was due to the fatigue of inspiratory muscles. Romer et al. (2002) defined this fatigue as the incapacity of muscles to develop sufficient contraction force, thus reflecting that the contraction properties of these muscles have been undermined.
The pathophysiological mechanisms that induce inspiratory muscle fatigue nevertheless remain unclear. The main hypotheses concern diaphragmatic cellular lesions (Romer and Polkey, 2008), the ventilatory requirements imposed by the intensity and duration of the exercise (Johnson et al., 1993), and neuromuscular junction dysfunction in the inspiratory muscles (Harms et al., 1998). After respiratory fatigue, a decrement in the alveolar ventilation and an increment in arterial CO2 occur, and the RM function is then unable to develop sufficient force to produce ventilatory muscle work (Roussos et al., 1979). During high-intensity exercise, the pulmonary load increases. This situation causes RM fatigue that cannot compensate for the tissue O2 demand and the athlete then feels respiratory fatigue (Smith et al., 2014). Other mechanisms may also be implicated in the alterations in lung volumes, with a significant effect on inspiratory muscle pressure. For example, we cannot exclude the hypothesis that increases in RV or decreases in FVC were responsible for the decrease in inspiratory muscle pressure (Boussana et al., 2003). Following exercise or 24 hr later, transdiaphragmatic pressure and inspiratory muscle pressure were reported to recover partially or almost completely (Ross et al., 2008). This observation argued for rapidly reversible pathophysiological mechanisms involved in the exercise-induced decrease in maximal inspiratory pressure.
In contrast, Perret et al. (1999) found no decrease in maximal inspiratory pressure after an exhaustive cycling endurance test performed at 85% VO2max in healthy subjects. This might suggest that, despite an exercise intensity that was reported to induce RM fatigue and exhaustion, the test duration—which was not indicated by the authors—was insufficient to generate a decrease in maximal inspiratory pressure. Several authors have shown a lack of modification in maximal expiratory pressure after endurance exercise (Chevrolet et al., 1993; Ross et al., 2008). In contrast, maximal expiratory pressure was found significantly decreased 20–60 min at the end of the marathon race (Loke et al., 1982). The authors suggested that this decrease was due to a markedly increased and prolonged ventilatory demand requiring inspiratory and expiratory muscle recruitment, particularly from the abdominal muscles. In our study, we measured maximal expiratory pressure (Fig. 1) 3:00±0:30 hr after the triathlon, and this difference in the time at which maximal expiratory pressure was measured might explain this difference.
Decreased RM endurance has been widely observed after strenuous sports activity. An earlier study reported this decrease in endurance as assessed by MVV after a marathon (Loke et al., 1982), and another study noted a significant decrease in Tlim for inspiratory muscles (26.5%) 3 days after completion of an ultramarathon (about 87 km) performed at 10.2 km/hr (Ker and Schultz, 1996). In contrast, Warren et al. (1989) reported no significant modification in MVV during and after a 24-hr ultramarathon performed at 6.3 km/hr. The authors assumed that the race speed was insufficient to solicit the respiratory apparatus at the level of an intense and short-duration activity. Exercise intensity may thus play a determinant role in RM fatigue (Bender and Martin, 1985). Romer and Polkey (2008) indicated that throughout intense exercise, the level of ventilation sustained by the RM causes fatigue in these muscles. Such RM fatigue could activate the metaboreflex, thus leading to vasoconstriction and a subsequent lack of nutrient supply to muscle and, ultimately, to muscle fatigue, which would limit performance in endurance exercise (Katayama et al., 2012; Wüthrich et al., 2013).
It should also be noted that the degree of fatigue can be influenced by the exercise modality. Hill et al. (1991) and Boussana et al. (2001) observed that cycling involved the participation of thoracic muscles more than diaphragmatic muscle, and this activity has been found to generate greater RM fatigue than running. Indeed, these results indicated that the relative brevity of an Olympic distance triathlon was accompanied by a relatively high intensity of activity, both conditions which would be favorable to high ventilation (Hue et al., 1999), and thus RM fatigue. In addition, the different exercise modalities and successions may have further contributed to the decline in RM performance (Boussana et al., 2001). Effectively, swimming involves the RM for ventilation with an active expiratory phase in the water and an inspiratory phase in combination with arm locomotion, and cycling has been reported to induce RM fatigue because of the crouched position and the increase in abdominal and thoracic impedance (Hill et al., 1991).
The observation of decreased endurance Tlim (Fig. 3) 24 hr after the race, in contrast to inspiratory muscle strength, raises questions about the pathophysiological mechanisms involved in maximal inspiratory pressure and RM endurance. The pathophysiological phenomena implicated in the decrease in maximal inspiratory pressure may be numerous and recover rapidly after the end of exercise, particularly altered pulmonary volumes (Johnson et al., 1993), bronchoconstriction, decreased pulmonary compliance, peribronchial oedema, and airways obstruction (Zavorsky et al., 2019). In contrast, the decrease in RM endurance has been suggested to result from a decrease in the glycogen reserve in the ventilatory muscles, a mechanism which requires several hours or days to recover completely (Bender and Martin, 1985; Chevrolet et al., 1993).
A consequence of inspiratory muscle fatigue is the reduction in oxygen transport and the amount of circulating blood to the working muscles, thus increasing the fatigue of the peripheral muscles and decreasing performance during endurance exercise (Akınoğlu et al., 2019; Katayama et al., 2012). RM has been reported to be skeletal muscle and morphologically and functionally similar to locomotor muscles (Chevrolet et al., 1993; Romer and Polkey, 2008; Romer et al., 2002). RM training improves whole-body endurance capacity (Verges et al., 2007), whereas RM fatigue may result in the inability to maintain gas and pH levels within an acceptable range, limiting exercise tolerance (Romer and Polkey, 2008). Similarly, Harms et al. (1998) reported an increase in respiratory work during maximal exercise, which induces vasoconstriction in the locomotor muscles and less perfusion to these muscles and VO2. We thus suggest that the persistent decrease in RM endurance on the day following the triathlon could be linked to lower recovery and might limit daily training performance.
We recommend that respiratory function should be monitored throughout the competitive season in triathletes and that specific inspiratory muscle training should be considered during triathlon training sessions. This may be important as it has been demonstrated that RM training increases the amount of time athletes can exercise and improves inspiratory muscle strength and endurance. To this end, it is necessary to set up other studies in order to link the specific training of the RMs and the improvement in sports performances.
Despite the reported limitations of using voluntary tests to measure the performance of RMs, they have now been validated and popularized (Ross et al., 2008). In our study, the athletes were familiar with the tests and motivated; they were also encouraged throughout the measurements. The observed decrease in the performance of the inspiratory muscles cannot be attributed to their lack of motivation. Although we did not measure the partial pressure of CO2, it has been consistently reported no change in previous studies (Gill et al., 2014; Matecki et al., 2001). In conclusion, we have shown that an Olympic distance triathlon causes a decrease in the strength and endurance of inspiratory muscles. Although inspiratory muscle strength recovered rapidly after the race, the persistent reduction in RM endurance 1 day following the triathlon expands the evidence of inspiratory muscle fatigue. However, it raises the questions of whether these are the same mechanisms that are involved in the fatigue of the RMs when it comes to the reduction in strength or endurance of RM, and also whether the fatigue of RMs is a limitation of sports performance.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.