Treadmill exercise improves spatial learning ability by increasing cell proliferation in offspring born to maternal rats receiving stress during pregnancy
Article information
Abstract
Prenatal stress causes learning deficits by inhibiting neurogenesis in the hippocampus. We studied the effects of maternal treadmill running or offspring treadmill running on the spatial learning ability of adolescent offspring rats or adult offspring rats born to maternal rats that received stress during pregnancy. For this study, spatial learning ability was measured by radial 8-arm maze task. Immunohistochemistry for 5-bromo-2′-deoxyuridine and Western blot for brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB), Bcl-2-associated X protein (Bax), and B-cell lymphoma 2 (Bcl-2) were also conducted. Stress was induced by exposing pregnant rats to hound in an enclosed room. Maternal treadmill running or treadmill running of offspring improved spatial learning ability of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring increased hippocampal cell proliferation of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring increased BDNF and TrkB expression in the hippocampus of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring inhibited Bax expression and increased Bcl-2 expression in the hippocampus of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Mother’s exercise during pregnancy or child’s exercise after childbirth can improve the spatial learning ability deteriorated due to stress during pregnancy.
INTRODUCTION
Prenatal stress causes learning deficits by inhibiting neurogenesis in the hippocampus (Lemaire et al., 2000). Prenatal stress causes dysfunction of the hypothalamic-pituitary-adrenal axis, leading to impairment in fetal brain development (Maccari et al., 2003). Prenatal noise exposure retarded growth, decreased hippocampal neurogenesis, and disturbed spatial learning ability in rat pups, in contrast, prenatal music exposure increased hippocampal neurogenesis and improved spatial learning ability in rat pups (Kim et al., 2006). Radial 8-arm maze task has been used to measure spatial learning ability (Ko et al., 2019).
Immunohistochemistry for 5-bromo-2′-deoxyuridine (BrdU) is used to quantify the generation of new cells, and the neuronal nuclear antigen is a remarkable marker for verifying neuronal maturity (Ji et al., 2020). The hippocampus is where nerve cells are produced and is an important brain area that is implicated in learning and memory.
Enhancement of neurogenesis was not seen in brain-derived neurotrophic factor (BDNF) knockout mice (Rossi et al., 2006). BDNF is implicated in the neuronal differentiation and survival by binding to the receptor tyrosine kinase B (TrkB), and BDNF secretion is regulated in an activity-dependent manner (Massa et al., 2010). BDNF signaling has an effect on neurotrophic support for neurons and also has an inhibitory effect on apoptosis (Neeley et al., 2011).
Apoptosis modulates tissue development and homeostasis. Manipulation of the B-cell lymphoma 2 (Bcl-2) family is helpful in the treatment of stroke and neurodegenerative diseases (Akhtar et al., 2004). The Bcl-2 family is divided into antiapoptotic proteins and pro-apoptotic proteins according to their function (Upadhyay et al., 2003). Bcl-2 inhibits cell death, whereas, Bcl-2-associated X protein (Bax) initiates cell death (Kuwana and Newmeyer, 2003).
We studied the effects of maternal treadmill running or offspring treadmill running on the spatial learning ability of adolescent offspring rats or adult offspring rats born to maternal rats that received stress during pregnancy. Spatial learning ability was evaluated by radial 8-arm maze task, and immunohistochemistry for BrdU and Western blot for BDNF, TrkB, Bax, and Bcl-2 were also done.
MATERIALS AND METHODS
Adolescent and adult rats
Adolescent and adult offspring Sprague-Dawley male rats were used for the experiment (Orient Co., Seoul, Korea). The pregnant rats were classified into the 3 following groups (n=5 per group): the control group, the stress-induced group, and the stress-induced and exercise group. Offspring of the stress-induced group were classified into offspring of maternal stress group and offspring with exercise of maternal stress group. As a result, adolescent (280± 10 g in weight, 12 weeks in age) and adult (320±10 g in weight, 24 weeks in age) offspring rats were randomly assigned into the 4 groups according to the status of maternal rats (n=7 in each group): offspring of control group, offspring of maternal stress group, offspring of maternal stress with exercise group, and offspring with exercise of maternal stress group. The pregnant rats received BrdU intraperitoneally (100 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) once a day, 30 min before treadmill exercise, and 3 times per week for 2 weeks. Offspring rats also received BrdU (50 mg/kg) intraperitoneally once a day, 30 min before treadmill exercises, for 2 weeks. For this experiment, approval number was achieved from the Institutional Animal Care and Use Committee of Tongmyong University (TMU-19-078).
Inducing stress in pregnant rats
Stress was induced by exposing pregnant rats to hound in an enclosed room. The exposure time to stress lasted for 10 min, repeated 3 times a day at 1-hr intervals. Exposure of maternal rats to hound continued from pregnancy to delivery. The reaction of pregnant rats to hounds was observed from a distance, which included approaching, sniffing, and sometimes chasing with mild attacks. The hound could approach the pregnant rat, but could not harm the pregnant rat. The same hound was used throughout the experiment.
Exercise protocol
Pregnant rats in the exercise groups ran on a motorized treadmill for 30 min once a day, starting 7 days after pregnancy and continued until delivery. Offspring rats in the exercise group ran a motorized treadmill for 30 min once a day for 4 weeks. Exercise load was applied by running at a slope of 0° at 2 m/min for the first 5 min, 5 m/min for the next 5 min, and 8 m/min for the last 20 min.
Radial 8-arm maze task
Spatial learning ability was done by a radial 8-arm maze device, as mentioned in the below (Ko et al., 2019). The radial 8-arm maze device consisted of a central octagonal plate (30 cm in diameter) and 8 radial arms (50 cm long, 10 cm wide), placed 1 m above the floor. There was a small container (3 cm in diameter and 1 cm in depth) filled with water at the end of the arm. The rats were deprived of water for 24 hr before training or testing, and the rats were able to explore and drink the water for 5 min. The rats were trained 3 times before taking the spatial learning ability test. On day 12 of the experiment, a spatial learning ability test was conducted. The time it took to find water at the tip of the arms was recorded, revisiting the previously visited arm was checked as error, and the visit before the first error was checked as correct. The experiment was finished when water was found in all 8-arms or 6 min of searching for water had passed.
Tissue preparation
After completing the radial 8-arm maze task, the rats were sacrificed mentioned in the below (Ko et al., 2019). Anesthesia was performed using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France) followed by infusion of 50 mM phosphate-buffered saline (PBS) through the heart. The rats were fixed with 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). Brains were extracted and fixed overnight, then transferred to 30% sucrose solution for preventing freezing. Using a freezing microtome (Leica, Nussloch, Germany), 40-μm thick coronal sections, average 10 sliced sections, were made from the hippocampus of each rat.
Immunohistochemistry for BrdU
BrdU immunohistochemistry was done, as mentioned in the below (Ji et al., 2020). After the sections were treated with 0.5% Triton X-100 in PBS for 20 min, incubated with 50% formamide-2 x standard saline citrate at 65°C for 2 hr, denatured in 2 N HCl for 30 min at 37°C, and washed twice in 100 mM sodium borate (pH, 8.5). Afterwards, the sections were incubated with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany) overnight at 4°C. After washing 3 times with PBS, the sections were treated with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 hr. The sections were treated with an avidin-peroxidase complex (1:100; Vector Laboratories) for another 1 hr, treated with 50 mM Tris-HCl (pH, 7.6) containing 0.02% diaminobenzide, 40-mg/mL nickel chloride, and 0.03% H2O2 in 50 mM Tris-HCL (pH, 7.6) for 5 min. BrdU-positive cells were confirmed by treating with a mouse antineuronal nuclear antibody (1:1,000; Chemicon International, Temecula, CA, USA). After washing 3 times with PBS, the sections were treated with a biotinylated anti-mouse secondary antibody for 1 hr, and then treated with a reaction mixture of 0.03% diaminobenzide with 0.03% H2O2 for 5 min for staining.
Western blot for BDNF, TrkB, Bax, and Bcl-2
Western blot was done, as mentioned in the below (Ji et al., 2020). The hippocampal tissues were homogenized using lysis buffer consisting of 1 mM EGTA, 1 mM PMSF, 1 mM Na2VO4, 1.5 mM MgCl2·6H2O, 50 mM Tris-HCl (pH, 8.0), 100 mM NaF, 150 mM NaCl, 1% Triton X-100, 10% glycerol, and then centrifuged at 50,000 rpm for 1 hr. Anti-β-actin antibody (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-BDNF (1:1,000), anti-TrkB (1:1,000), anti-Bax antibody (1:1,000; Santa Cruz Biotechnology), and anti-Bcl-2 antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for β-actin, Bax, and Bcl-2 (1:3,000; Amersham Pharmacia Biothech GmbH, Freiburg, Germany), and horseradish peroxidase-conjugated anti-rabbit antibody for BDNF and TrkB (1:5,000; Vector Laboratories), were used as the secondary antibodies. In addition to the membrane transfer performed at 4°C, all other steps were performed at room temperature. Band detection was done by enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
BrdU-positive cell number in the hippocampal dentate gyrus was calculated hemilaterally and presented as the number of cells per mm2. The detected band was measured with a densitometer. One-way analysis of variance followed by Duncan post hoc test was used for data analysis, and the data were presented as the mean± standard error of the mean. Statistically significance was determined at P<0.05.
RESULTS
Spatial learning ability
The results of radial 8-arm maze are shown in Fig. 1. Spatial learning ability of adolescent and adult offspring rats was deteriorated by stress during pregnancy. Maternal treadmill running during pregnancy or treadmill running of offspring restored learning ability of adolescent and adult offspring rats.

Spatial memory of radial 8-arm maze in adolescent (I) and adult (II) offspring rats. Left panel: correct choice number. Right panel: error number. A, offspring of control group; B, offspring of maternal stress group; C, offspring of maternal stress with exercise group; D, offspring with exercise of maternal stress group. The results are expressed as the mean±standard error of the mean. *P<0.05 compared with offspring of control group. #P<0.05 compared with offspring of maternal stress group.
Cell proliferation
The results of BrdU immunohistochemistry are shown in Fig. 2. Cell proliferation of adolescent and adult offspring rats was suppressed by stress during pregnancy. Maternal treadmill running during pregnancy or treadmill running of offspring enhanced cell proliferation of adolescent and adult offspring rats.
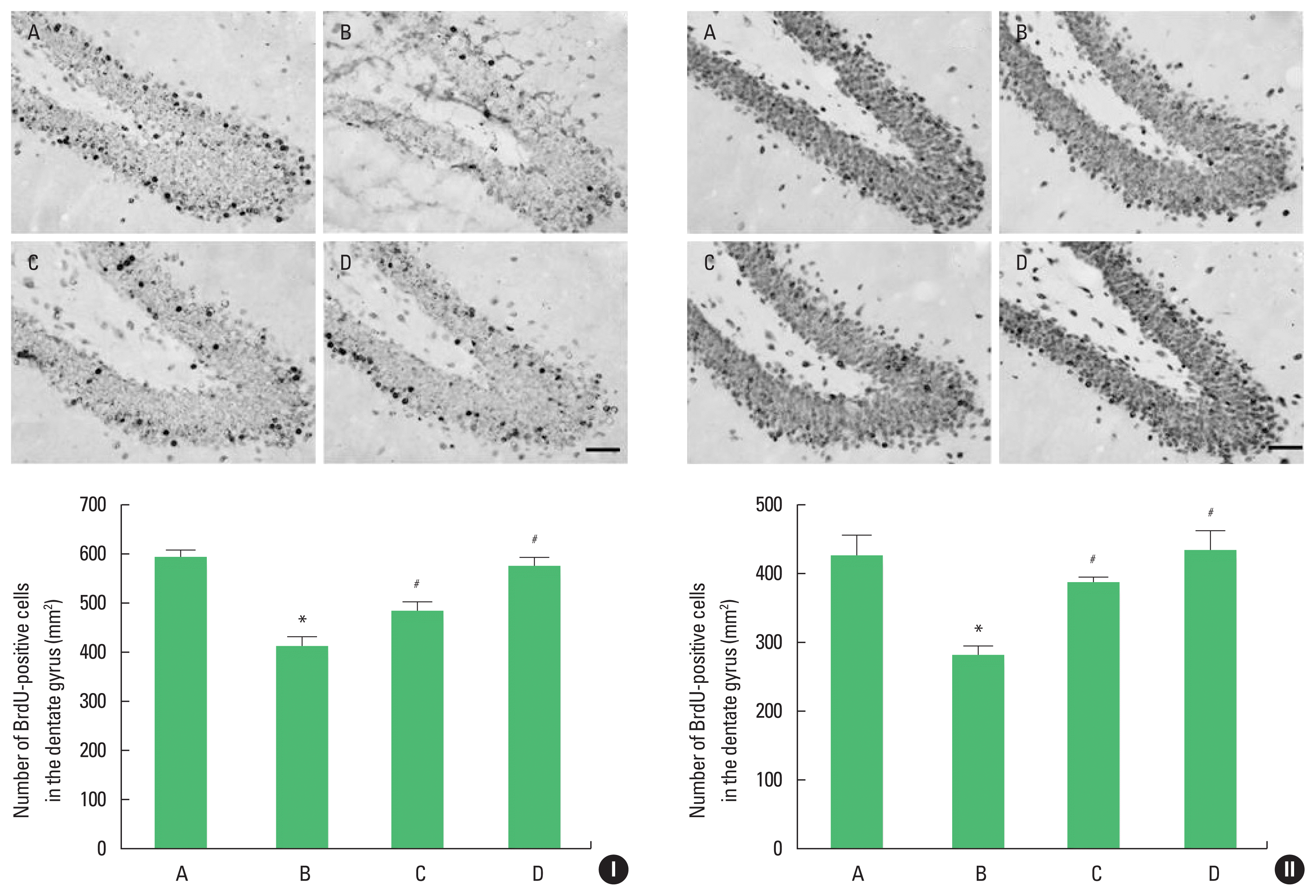
Cell proliferation in the hippocampal dentate gyrus of adolescent (I) and adult (II) offspring rats. Upper panel: photomicrograph of 5-bromo-2′-deoxyuridine (BrdU)-positive cells. The scale bar represents 50 μm. Lower panel: number of BrdU-positive cells in each group. A, offspring of control group; B, offspring of maternal stress group; C, offspring of maternal stress with exercise group; D, offspring with exercise of maternal stress group. The results are expressed as the mean±standard error of the mean. *P<0.05 compared with offspring of control group. #P<0.05 compared with offspring of maternal stress group.
BDNF and TrkB expression
The results of Western blot analysis for BDNF and TrkB expression in the hippocampus are shown in Fig. 3. BDNF and TrkB expression of adolescent and adult offspring rats was decreased by stress during pregnancy. Maternal treadmill running during pregnancy or treadmill running of offspring increased BDNF and TrkB expression of adolescent and adult offspring rats.
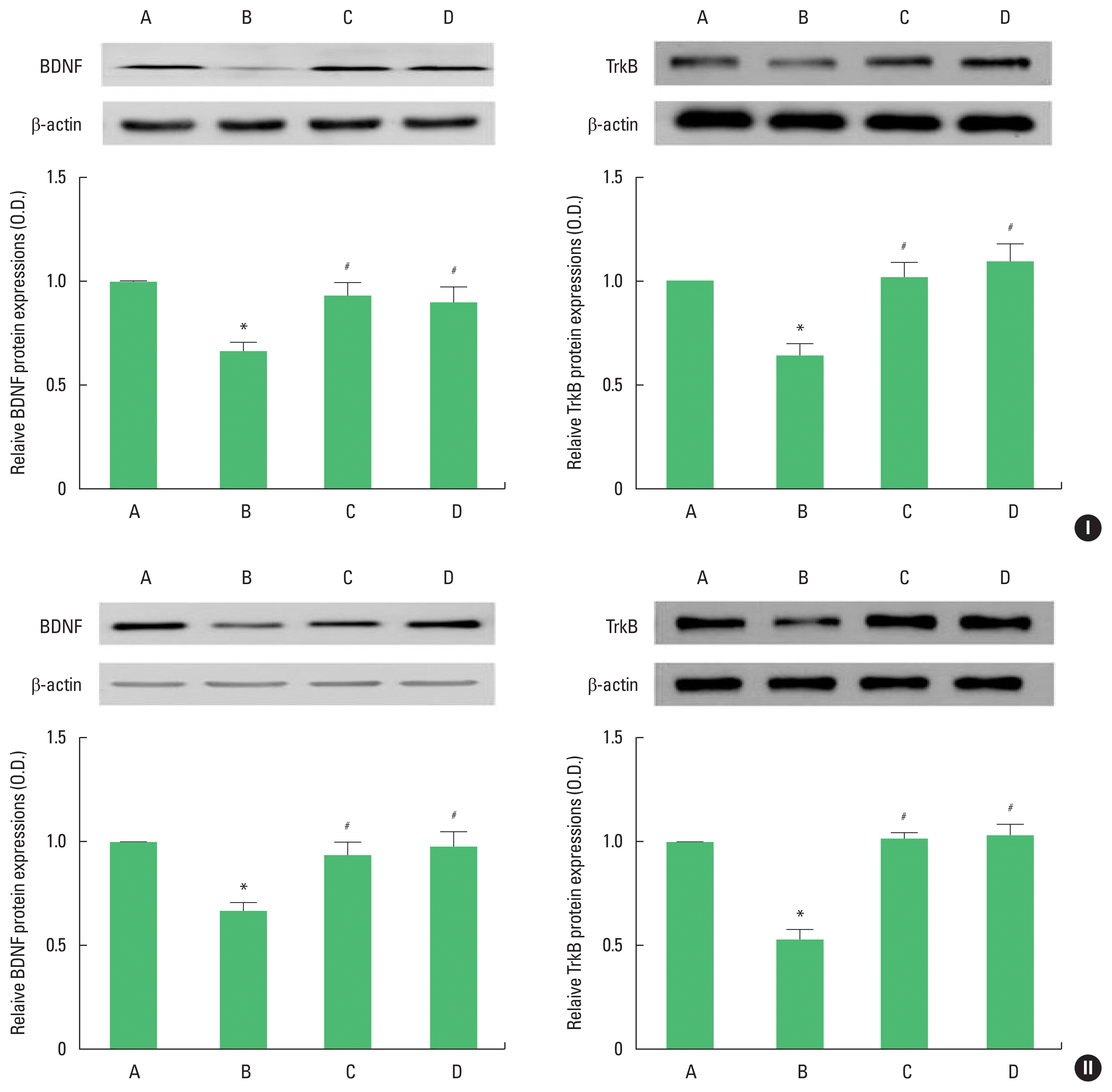
Brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) expression in the hippocampus of adolescent (I) and adult (II) offspring rats. Upper panel: representative expression of BDNF or TrkB. Lower panel: expression of BDNF or TrkB in each group. A, offspring of control group; B, offspring of maternal stress group; C, offspring of maternal stress with exercise group; D, offspring with exercise of maternal stress group. The results are expressed as the mean±standard error of the mean. *P<0.05 compared with offspring of control group. #P<0.05 compared with offspring of maternal stress group.
Bax and Bcl-2 expression
The results of Western blot analysis for Bax and Bcl-2 expression in the hippocampus are shown in Fig. 4. Bax expression was enhanced and Bcl-2 expression was inhibited in adolescent and adult offspring rats by stress during pregnancy. Maternal treadmill running during pregnancy or treadmill running of offspring was inhibited Bax expression and Bcl-2 expression was increased in adolescent and adult offspring rats.
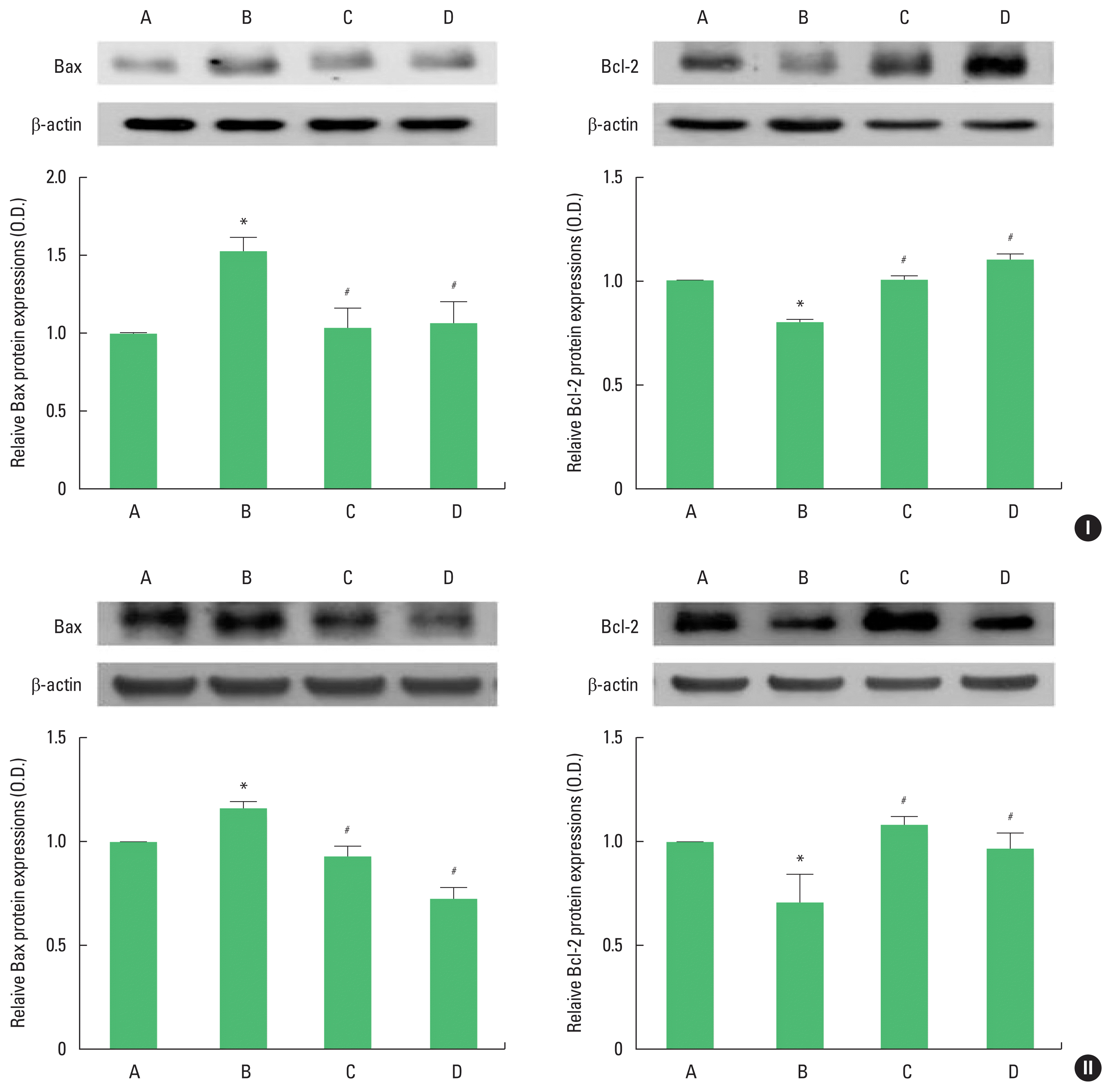
Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2) expression in the hippocampus of adolescent (I) and adult (II) offspring rats. Upper panel: representative expression of Bax or Bcl-2. Lower panel: expression of Bax or Bcl-2 in each group. A, offspring of control group; B, offspring of maternal stress group; C, offspring of maternal stress with exercise group; D, offspring with exercise of maternal stress group. The results are expressed as the mean±standard error of the mean. *P<0.05 compared with offspring of control group. #P<0.05 compared with offspring of maternal stress group.
DISCUSSION
Early adversity can exacerbate amygdala and hippocampal dysfunction, and early intervention can mitigate early adversity-mediated reinforcement of hippocampal dysfunction (Imanaka et al., 2006). Maternal swimming during pregnancy enhanced short-term memory with increasing neuronal proliferation in the hippocampus of rat pups (Lee et al., 2006). Maternal treadmill running during pregnancy improved the short-term memory with increasing cell proliferation in the hippocampus of rat pups (Kim et al., 2007). Stress during pregnancy impaired the development of the fetus’ brain, which has serious neurobiological effects after birth, leading to cognitive deficits (Kapoor et al., 2009). In the current results, spatial learning ability was impaired in adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring improved spatial learning ability of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy.
Prenatal stress suppressed hippocampal cell proliferation in rats throughout their lifespan, suggesting that early stress exposure accelerated the age-related reduction in hippocampal plasticity (Lemaire et al., 2000). Wheel exercise increased hippocampal cell proliferation in socially housed rats, however, isolation prevented this running-induced increase of cell proliferation (Leasure and Decker, 2009). The chronic mild stress presented depression-like behaviors with inflammation, neuronal cell damage, and suppressed neurogenesis and apoptosis in the hippocampus (Kubera et al., 2011). In the current results, hippocampal cell proliferation was impaired in adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring increased hippocampal cell proliferation of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy.
BDNF enhanced resistance to nerve injury, improved neuronal survival (Mizuno et al., 2000), and modulated neuronal generation in the hippocampus (Donovan et al., 2008). Neeley et al. (2011) showed that prenatal stress decreased BDNF and TrkB expression in the hippocampus. Treadmill running during pregnancy increased BDNF and TrkB expressions in rat pups born to obese maternal rats (Ji et al., 2020). They concluded that the effect of improving short-term memory by treadmill running could be attributed to enhanced neurogenesis through BDNF-TrkB activation by treadmill running (Ji et al., 2020). In the current results, BDNF and TrkB expression was decreased in the hippocampus of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring increased BDNF and TrkB expression in the hippocampus of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy.
Bcl-2 overexpression improved neurogenesis and neuronal survival, suggesting that increased neurogenesis prevented the death of neonatal neurons (Zhang et al., 2006). Treadmill exercise inhibited expression of Bax and enhanced expression of Bcl-2 in the brain-injured rats (Kim et al., 2010). Lee et al. (2011) reported that maternal exercise activated Bcl-2 and inhibited Bax expression in the hyperthermia-induced apoptosis of the embryo. In the current results, Bax was enhanced and Bcl-2 was suppressed in adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy. Maternal treadmill running or treadmill running of offspring inhibited Bax expression and increased Bcl-2 expression in the hippocampus of adolescent and adult offspring rats born to maternal rats receiving stress during pregnancy.
Through this experiment, it was proved that the mother’s exercise during pregnancy or the child’s exercise after childbirth can improve spatial learning ability when stressed during pregnancy. The effect of this exercise was achieved by hippocampal cell proliferation caused by enhancement of BDNF expression and inhibition of apoptosis.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018S1A5A2A01038919).
