Traditional Thai exercise (Ruesi Dadton) for improving motor and cognitive functions in mild cognitive impairment: a randomized controlled trial
Article information
Abstract
This study determined the effectiveness of a 12-week cycle of Ruesi Dadton (RSD) among older adults with mild cognitive impairment (MCI), for improving cognitive and physical performance. Seventy-six participants were included and were divided equally into two groups. A group performed RSD exercise for 60 min, 3 times/wk for 12 weeks, and the control group did not perform RSD exercise. The primary endpoint was cognitive function, as assessed by the Montreal cognitive assessment (MoCA), Mini-Mental State Examination, verbal fluency (VF) test, and trail making test parts A and B (TMT-A and TMT-B). The secondary endpoints were the Timed Up and Go (TUG) test, handgrip, and gait speed results, which were used to evaluate the physical function. There were significant differences in the TMT-B and handgrip scores (P<0.05) between the two groups. Both groups had improved MoCA scores (P<0.05) and normal walking speeds (P<0.01). Additionally, the RSD group showed improved VF test (P<0.01), TMT-B (P<0.01), and TUG test (P<0.05); a negative correlation was found between MoCA and TUG test (P<0.05). However, high walking speed and handgrip (P<0.05) worsened in the control group. RSD exercise resulted in relevant improvements in the cognitive and physical functions in MCI.
INTRODUCTION
Mild cognitive impairment (MCI) can be indicative of Alzheimer disease (AD) or other forms of dementia. During a follow-up of patients with MCI, progression of MCI to dementia occurred in 29% of participants, of which 81% was thought to be caused by underlying AD (Hanfelt et al., 2018). The Montreal cognitive assessment (MoCA) and Mini-Mental State Examination (MMSE) are used to determine the standardised differential diagnosis of MCI (Nasreddine et al., 2005; Petersen et al., 2001). The primary characteristic of MCI is brain atrophy, leading to cognitive decline, that usually occurs with age but is more than expected; however, the cognitive decline is not as rapid as it is in AD (Gheysen et al., 2018).
Molecular inflammation leading to functional decline and pathological aging such as that accompanying dementia can be modulated by calorie restriction and exercise (Chung et al., 2009). The main problems in gerontology include physical and cognitive decline that occurs with advancing age (Ma and Chan, 2020). The imbalance in redox systems causes this molecular inflammation that results in multisystemic functional impairment, such as in the musculoskeletal and neurological systems, ultimately leading to disability (El Assar et al., 2020). Accordingly, persons with a low level of physical activity have a corresponding increased rate of memory loss (Nemoto et al., 2018). Moreover, physical inactivity during later life creates a greater risk of dementia (Livingston et al., 2017). The mechanism of physical exercise can promote brain proliferation by evaluating BDNF and irisin (Jin et al., 2018). There is strong evidence to suggest that continuous physical activity decreases the risk of dementia (Baumgart et al., 2015). Moreover, exercise is a nonpharmacological and therapeutic approach that can prevent the progression of MCI to dementia (Petersen et al., 2018). Persons with MCI who continue performing physical activity have been found to have improved cognitive function (Gheysen et al., 2018). High level of physical activity is also associated with low risk of MCI and dementia of any type (Laurin et al., 2001). The physical performance not only relates to cognitive function in older adults but is also utilized to predict frailty and sarcopenia such as grip strength, TUG test, and walking speed (Keevil and Romero-Ortuno, 2015). Therefore, individuals with dementia are recommended to exercise during all stages of the disease and in many combinations, to improve their strength, balance, mobility, and ability to perform activities of daily living (ADL) (Blankevoort et al., 2010).
Mind-body exercises are beneficial to persons with MCI (Brenes et al., 2019; Eyre et al., 2017; Sungkarat et al., 2017); however, a recent study found that mind-body intervention had a small effect and relatively low certainty for the cognitive function of individuals with MCI (Demurtas et al., 2020). Hence, further confirmation of such results is needed. Ruesi Dadton (RSD) is a traditional Thai exercise that involves slow movement with deep breathing and breath-holding for well-being particularly in the elderly (Noradechanunt et al., 2017), similar to what is performed in a multimodal exercise program. RSD is also a type of mind-body exercise, just like yoga and Tai Chi. Moreover, a previous study had also reported that multimodal exercises concurrent with high-intensity mental activity can promote physical and cognitive performance (Canli and Ozyurda, 2020). In another study, individuals whose occupation involved working on computers showed significant improvement in motion and a tendency toward improved cognition on performing RSD exercise for 4 days (Tanasugarn et al., 2015). However, there is insufficient evidence regarding the ability of RSD exercise to improve cognitive and physical functions in MCI. We hypothesised that RSD exercise enhances cognitive and physical performance. Therefore, this study aimed to examine the effects of 12 weeks of RSD on cognitive and physical performance in MCI.
MATERIALS AND METHODS
Study design
This randomized controlled trial blinded assessors into RSD and control groups. Fig. 1 demonstrates the flow of the study. Ethical approval was obtained from The Human Research Ethics Committee No. 1, Faculty of Medicine, Thammasat University, Thailand (no. 119/2562).
Participants
The participants were recruited at Huadon Health Promoting Hospital, Ubon Ratchathani Province, Thailand, between June and July 2020. A total of 274 individuals aged 50–80 years were included and grouped according to their ability to read and write (Geda et al., 2010; Northey et al., 2018), hearing ability, speaking ability, ability to communicate in Thai, cognition issues (Petersen, 2004), body mass index (BMI; low to moderate risk, 19–27.5 kg/m2) (WHO Expert Consultation, 2004), MMSE score (≥24 points or <24 points) (Mitchell et al., 2014), MoCA score (<26 points or ≥26 points) (Nasreddine et al., 2005; Petersen et al., 2001), and diagnosis of amnestic MCI by a neurologist. The exclusion criteria were as follows: dementia; history or diagnosis of cardiovascular disease; neurological disease or mental symptoms; blood pressure >160/100 mmHg; history of alcoholism or drug addiction; spine problems; history of brain accident or injury; knee pain (visual analogue scale score ≥8) or the inability to sit cross-legged; a severe accident during the 3 months before study enrolment; continued regular exercise within the past 2 years (≥30 min/day, ≥3 days/wk); and the intent to perform exercise such as RSD, Tai Chi, yoga, or Chi-Qong within 3 months of study enrolment.
Sample size
A sample size program was used to calculate the number of participants using T statistics to generate the following two-tailed values: α=0.05; β=0.2; q1=0.5; q0=0.500; E=0.66; and S=1 (Huang and Chow, 2019). The standard normal deviation for α was 1.96, the standard normal deviation for β was 0.84, and the standardised effect size (ES) was 0.62 (Kohn and Senyak, 2021). The total number of participants needed was 66; however, an additional 15% participants were recruited in case of dropout. Therefore, recruitment was complete when 76 participants were enrolled.
Randomization
A random allocation of participants in each group was operated by randomized block sizes stratified for age and years of formal education. The first criterion for stratification was advanced age because of its association with MCI (Gheysen et al., 2018). The second criterion for stratification was reduced duration of education, which highly contributes to risk of dementia (Livingston et al., 2017).
Blinding
This trial adopted a single-blinded assessment owing to the nature of experimental protocol; thus, the participants were aware of their group allocation. The data were collected by assessors who were blinded to the group allocation; group numbers were not indicated on participant forms.
Intervention
Eligible participants were enrolled in the study after screening, and baseline characteristics were recorded. Before the intervention, both groups were provided with information regarding MCI and dementia prevention by the neurologist. The RSD group participated in 2 days of RSD exercise practice; afterward, they started performing RSD exercise using an instruction video for 12 weeks (60 min/session, 3 times/wk). Two Thai medicine instructors led RSD practices for over a period of 2 days; the first day involved breathing exercises and movement corrections, while the second day emphasised deep breathing and breath-holding with slow movements while performing RSD. The researcher (PK) thoroughly supervised the said program. The video included 15 postures with 10 repetitions for each posture according to the performance guidelines of the Thai Ministry of Public Health (Khanthong et al., 2021).
The RSD interventions were conducted after completion of all measurements at baseline. RSD videos, exercise models before a class, supervisor guidance, and 2–4 supervisor assistants were involved in the morning program between 8:00 and 10:00 a.m.
Cognitive tests
The MoCA and MMSE global cognitive tests were used for screening; individuals with MCI with scores of ≥24 and <25 were asked to participate in the study (Nasreddine et al., 2005). The verbal fluency (VF) test score was used to determine memory function; individuals were asked to name as many animals as possible within 1 min (Muangpaisan et al., 2010). The trail making test (TMT) was used to determine executive function: TMT part A (TMT-A) involved drawing a line of numbers from 1 to 25 inside a circle and TMT part B (TMT-B) involved drawing lines from numbers to months inside a circle (in Thai abbreviated language) (Tombaugh, 2004). The results were recorded as seconds using a stopwatch.
Physical tests
Balance, strength, and speed were measured using the Timed Up and Go (TUG) test, digital hand dynamometer (EH101; Camry, Guangdong, China), and walking speed, respectively, and were analyzed to determine physical function. The TUG test was used to evaluate agility and dynamic balance from sitting to standing, walking a distance of 3 m, turning around, and sitting in the same place. To measure handgrip strength, the participants sat face-to-face with the physical therapist and flexed their elbows at 90° with mild wrist extension in a neutral position. The participants were subsequently instructed to squeeze the handle of the dynamometer for 5 sec and, followed by a 2-min rest. Walking speed was measured using a 10-m normal walking speed (NWS) and 5-m high walking speed (HWS) tests conducted by three assessors (an instructor, a timer, and a signal starter).
Data analysis
Data were analyzed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Descriptive statistics of the frequency, percentage, mean, and standard deviation were used to analyze the data of the RSD and control groups. Comparisons of differences between the groups were performed using chi-square and Fisher exact tests for nominal scales, and the Mann–Whitney U-test for interval scales. The McNemar and Wilcoxon signed-rank tests were used to analyze differences within each group before and after the intervention using nominal and interval scales, respectively. The Pearson correlation test was used to determine the relationships of MoCA with cognitive function and physical performance.
RESULTS
Baseline characteristics
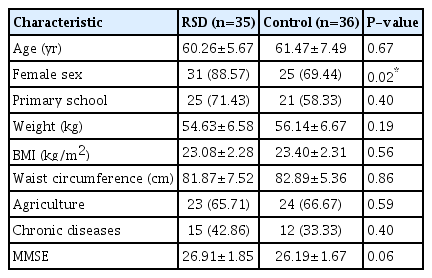
Seventy-one (RSD group, n=35; control group, n=36) participants were assessed during the analysis. Significant differences were not observed in terms of age, education, BMI, waist circumference, occupation, and illness history of the participants; however, sex was significant (P<0.05) (Table 1). With the exclusion of the dropout participants, this study has an 80.0% power to detect an ES of 0.665 (Kohn and Senyak, 2021).
Clinical assessments
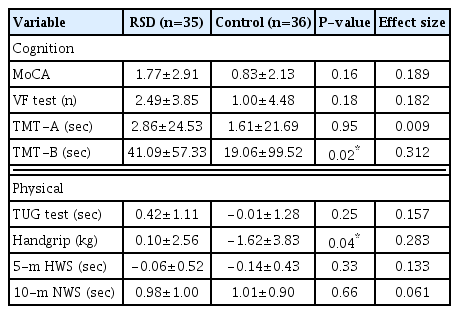
MoCA scores were significantly improved in both the RSD and control groups at P<0.001 and 0.02, respectively (Table 2). The RSD group also showed significant improvements at P<0.01 for VF test, TMT-B, and 10-m NWS, and at P<0.05 for TUG. The control group showed significant improvement in 10-m NWS scores at P<0.01, but the handgrip test and 5-m HWS scores were significantly worse than before (P<0.05).
When the results between the two groups were compared, significant differences in TMT-B (P=0.02) and handgrip (P=0.04) were observed (Table 3). A medium ES was observed in TMT-B, while others exhibited low association.
Correlations among changes in the MoCA scores and physical function
Changes in the MoCA scores based on the physical function were evaluated (Table 4). Negative correlations between the MoCA and TUG test scores (r=−0.38, P<0.05) were observed in the RSD group.
DISCUSSION
This study suggests that a 12-week RSD exercise program can induce significant improvements in physical function and reduce cognitive decline. The duration of this study was based on the suggestion of the World Health Organization that older adults (≥65 years) should perform at least 150 min of moderate exercise every week (Bull et al., 2020). The physical and cognitive performance results of this study were similar to those of the multimodal exercise program (Canli and Ozyurda, 2020). They were consistent with improved cognitive function observed in individuals aged 50 years who exercised at moderate intensity for 45–60 min per session (Geda et al., 2010; Northey et al., 2018); this also supports the idea that RSD exercise improves the cognitive function of healthy individuals within a short time (Tanasugarn et al., 2015). Participants were allocated to one of two groups based on their risk of MCI determined by age and education level (Luck et al., 2010). Although the baseline characteristics of both groups showed a significant difference in sex, this conflicted with the result of other studies on risk factors for MCI (Baumgart et al., 2015).
For cognition, evaluation of the MoCA scores indicated significant improvements in both groups; this can be attributed to various factors. For example, some MoCA questions (such as subtract 7 from 100) were difficult to answer for a few individuals. Another reason was high sensitivity of the questionnaire, which may have increased the scores (Nasreddine et al., 2005). The TMT-B representing the executive function revealed significant differences between and within groups because higher-complexity tasks require minimal changes to be detectable. Additionally, the results showed no significant difference in RSD, suggesting that TMT-A had simpler tasks than TMT-B. Furthermore, the VF test (representing memory function) scores were significant in the RSD group. These findings indicate that any type of mind-body intervention, such as Tai Chi, can reduce cognitive decline, especially in executive and memory functions of individuals with MCI and dementia (Gheysen et al., 2018; Nuzum et al., 2020). However, meta-analysis and systematic review of Tai Chi in MCI reported a different impact on cognitive results (Miller and Taylor-Piliae, 2014; Wayne et al., 2014; Wu et al., 2019). Wu et al. (2019) demonstrated that Tai Chi can improve global cognition and memory, but not executive function. Other reviews of Tai Chi found that the executive function can be improved (Miller and Taylor-Piliae, 2014; Wayne et al., 2014). Yoga also resulted in improvements in MCI when compared with the gold standard of memory training and executive function (Eyre et al., 2017).
RSD exercise can induce as much improvement in cognition, and executive and memory functions as shown by any other mind-body intervention. The results of the TUG, handgrip strength, and gait speed tests had varying consistency that depended on the duration, movement, posture, and intensity of the mind-body intervention. The TUG test showed significant improvements in the RSD group but not in the control group (Table 2). As a result, the TUG test measures distinct brain activity within the grey matter that is involved with many brain areas and differs between healthy individuals and individuals with MCI (Allali et al., 2020). This correlation may be significant because the task for TUG test is related to the medial temporal lobe, which is the area responsible for cognitive function (Kose et al., 2016). Multimodal exercise has been investigated, but significant improvements were not observed in the exercise group (De Andrade et al., 2013). Although mind-body intervention was a limitation in a number of related studies, significant differences were seen (Hishikawa et al., 2019; Hsieh et al., 2018). Virtual reality-based Tai Chi exercise resulted in many improvements in the physical function, and the control group experienced no significant changes (Hsieh et al., 2018). Individuals with MCI who practiced yoga and other exercise showed improvements in the TUG test score after 1 year but not within 6 months (Hishikawa et al., 2019).
Significant differences in handgrip strength between individuals with and without decline in cognitive function have been observed. Our data showed a significant decrease in the control group but not in the RSD group, and found a significant difference in strength between the groups. These results were similar to those of a previous study on Tai Chi (Sun et al., 2015) and another study on yoga (Ikai et al., 2017) that found significantly improved handgrip strength. Additional investigations of improvements in strength as a result of mind-body intervention are required.
For gait speed, the 5-m HWS score could possibly may be a more of a sensitive detector than the 10-m NWS score because as no significant decrease in the 5-m HWS score was found in the RSD group and while a significant decrease with a medium ES was detected in the control group. Our results were consistent with those of Sun et al. (2015) wherein the difference between groups after 3 months was not significant. Although there was a slight tendency for decreased 5-m HWS scores, which are consistent with those of a previous study, they were not observed within 3 months; however, they were observed after 6 months of performing Tai Chi (Hsieh et al., 2018; Sun et al., 2015). Therefore, longer follow-up periods are required to confirm the plausibility of these results. In the control group, reducing the speed of cognitive decline, which is a predictor of MCI and dementia, seemed to reverse dementia (Grande et al., 2020). Furthermore, another study found that the gait speed of individuals with diabetes could be a predictor and detector of MCI (Machii et al., 2020).
In conclusion, RSD, or traditional Thai exercise, has the potential to improve cognitive function and physical performance and reduce the risk of cognitive decline associated with any type of dementia, especially MCI. To the best of our knowledge, this is the first randomized controlled study on the effects of RSD exercise on individuals with MCI, that also examined cognitive and physical functions.
There were several limitations to this study. First, there was no screening for temporary MCI diagnoses, such as vitamin D and thyroid deficiency testing. Second, we could not limit confounding factors such as ADL and occupational activity performed by both groups. One participant in the control group experienced improvements in all parameters because of changes in behaviour (improved diet and increased exercise) upon being diagnosed with hypertension. Finally, there were limitations to the quality and quantity of mind-body exercise, particularly RSD exercise, performed by individuals with MCI. This study provides preliminary evidence that RSD exercise may be relevant in the prevention of cognitive decline, particularly for complex tasks, in the context of MCI. Furthermore, RSD exercise might lead to improvements in memory, balance, and speed; however, a larger study is required to confirm these results.
ACKNOWLEDGMENTS
The authors wish to thank the Thai Traditional Medical Knowledge Fund and the Thai Ministry of Public Health for funding this study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest to this article was reported.