Role of exercise in estrogen deficiency-induced sarcopenia
Article information
Abstract
A decline in estrogen levels during menopause is associated with the loss of muscle mass and function, and it can accelerate sarcopenia. However, with the growing number of postmenopausal women due to the increase in life expectancy, the effects of estrogen on skeletal muscle are not completely understood. This article reviews the relationship between estrogen deficiency and skeletal muscle, its potential mechanisms, including those involving mitochondria, and the effects of exercise on estrogen deficiency-induced skeletal muscle impairment. In particular, mitochondrial dysfunction induced by estrogen deficiency accelerates sarcopenia via mitochondrial dynamics, mitophagy, and mitochondrial-mediated apoptosis. It is well known that exercise training is essential for health, including for the improvement of sarcopenia. This review highlights the importance of exercise training (aerobic and resistance exercise) as a therapeutic intervention against estrogen deficiency-induced sarcopenia.
INTRODUCTION
The global population of older women (≥65 years) is increasing with an increase in life expectancy to 100 years. Menopause typically occurs between 49 and 52 years of age (Maltais et al., 2009), during which menstrual periods stop permanently. Thus, elderly women spend more than one-third of their lives in the postmenopausal stage, which is characterized by low levels of estrogen. Estrogen is known to play a crucial role in human physiology, including in glucose and lipid metabolism, bone metabolism, reproductive function, and neurological functions (Yonezawa et al., 2012). It is well accepted that the decline in circulating estrogen levels or estrogen deficiency is associated with central obesity, cardiovascular disease, type II diabetes, and osteoporosis (Brown et al., 2010; Klein-Nulend et al., 2015; Vogel et al., 2013). On the other hand, endogenous estrogen status in postmenopausal women was related to adiposity (Key et al., 2015), contributing to postmenopausal breast cancer.
Sarcopenia is defined as the loss of muscle mass, muscle strength, and physical performance (Cruz-Jentoft et al., 2019), which is a crucial precursor to frailty, leading to disability and the loss of independence (Fielding et al., 2011). Sarcopenia begins at the age of 30 years, and muscle mass decreases approximately 2%–7% every 10 years, which accelerates after the age of 60 years (Dodds et al., 2015). Previous studies have shown that a decrease in muscle mass is associated with a reduction in muscle strength and function (McPhee et al., 2018). Recently, however, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) indicated low muscle strength as a major determinant of sarcopenia (Cruz-Jentoft et al., 2019). Therefore, it is no longer sufficient to explain sarcopenia based on a decrease in muscle mass alone. The criterion method for measuring body composition, especially appendicular skeletal muscle, include bioelectrical impedance analysis, dual-energy x-ray absorptiometry (DXA), computed tomography, and magnetic resonance imaging (Messina et al., 2018; Vitale et al., 2021). In addition, muscle strength is usually measured using grip strength and the chair stand test (Cruz-Jentoft et al., 2019). Multiple mechanisms are related to the pathogenesis of sarcopenia, including motor neuron loss, dysregulation of cytokine secretion, increased inflammation, and mitochondrial dysfunction (Kim and Choi, 2013).
In postmenopausal women, decreased circulating estrogen levels directly or indirectly influence skeletal muscles and also accelerate the pathogenesis of sarcopenia (Khadilkar, 2019). After menopause, muscle mass declines by 0.6% per year (Rolland et al., 2007). In addition, estrogen plays an important role in the mitochondrial function (Ventura-Clapier et al., 2019). These effects of estrogen can be mediated through estrogen receptors (ERs), which are present in all cell types. Decreased estrogen levels and ERs result in mitochondrial dysfunction, altered mitochondrial dynamics, and diminished mitophagy (Ribas et al., 2016) and they induce mitochondria-mediated apoptosis (Boland et al., 2008). Estrogen deficiency may also induce muscle atrophy via mitochondrial dynamics, mitophagy, and mitochondria-mediated apoptosis.
It is well known that exercise is essential for health given its association with reduction of body fat, increase in muscle mass, and improvement in muscle strength, immune function, and the cardiovascular system. Accordingly, exercise training should be considered as a therapeutic intervention for estrogen deficiency-related sarcopenia. However, most studies have been conducted on the effects of hormone replacement therapy (HRT) on skeletal muscle function in postmenopausal women or ovariectomized (OVX) rodents. Therefore, in this review, we briefly describe the role of estrogen in skeletal muscle and the effects of exercise on estrogen deficiency-induced sarcopenia. We hypothesize that decreased estrogen levels lead to the deterioration of skeletal muscle, and exercise training ameliorates estrogen deficiency-induced sarcopenia.
SARCOPENIA AND MENOPAUSE
Skeletal muscles can be impaired by aging, loss of nutrition, disuse, motor neuron loss, dysregulation of cytokine secretion, and changes in hormone levels. The age-related loss of skeletal muscle mass and function is called “sarcopenia” (Cruz-Jentoft et al., 2019), which leads to adverse health-related outcomes, including frailty, cachexia, osteoporosis, metabolic syndromes, and death (Yoo et al., 2018). Rosenberg understood early sarcopenia in 1989 only as a decrease in muscle mass. However, in recent years, it has been defined as the loss of muscle function and muscle mass (Cruz-Jentoft et al., 2019). Moreover, the EWGSOP2 updated the definition of the criteria for age-related sarcopenia and proposed the loss of muscle mass and function (such as strength and performance) as an essential feature of sarcopenia (Cruz-Jentoft et al., 2019). Appendicular skeletal muscle mass (ASM) is usually measured using DXA, and the cutoff points of ASM for low muscle mass are <20 kg and <15 kg for men and women, respectively. Muscle strength and physical performance are usually measured using grip strength and gait speed, respectively. The cutoff points for diagnosing sarcopenia by grip strength are <16 kg for women and <27 kg for men, and gait speed is ≤0.8 m/sec for both men and women. An algorithm that can divide the level of sarcopenia (i.e., probable sarcopenia, sarcopenia, and severe sarcopenia) using the above cutoff points was newly presented by EWGSOP2.
Sarcopenia may be caused by several mechanisms, such as mitochondrial dysfunction, activation of inflammation, inadequate nutrition, loss of satellite cells, and hormonal changes (Cruz-Jentoft et al., 2010). In particular, there is a growing interest in studying the relationship between sarcopenia and hormones, especially estrogen changes. Life expectancy is increasing, and women spend more than 30 years in postmenopausal conditions. Menopause is defined as the permanent cessation of menstruation resulting from the loss of ovarian activity. Postmenopausal women with a natural or unnatural menopausal status, such as those with bilateral OVX, have low levels of estrogen, which could lead to a decrease in muscle mass and muscle strength (Carville et al., 2006; van Geel et al., 2009). The level of total body potassium, a marker of lean body mass, has been shown to significantly decrease during the first few years of menopause (Aloia et al., 1991). Moreover, a previous study suggested that postmenopausal women have an increased risk of sarcopenia (Messier et al., 2011); therefore, the decline of estrogen during the menopause is thought to be implicated in sarcopenia.
ESTROGEN AND THE SKELETAL MUSCLE
Estrogen is the primary female sex hormone secreted by the ovaries. Estrogen is involved in the differentiation, proliferation, and physiological functions of reproductive organs, such as the uterus, vagina, oviduct, and mammary glands (Ikeda et al., 2019). Moreover, estrogen plays an important role in the skeletal muscle, cardiovascular, and immune systems, as well as in metabolism (Muramatsu and Inoue, 2000; Nilsson and Gustafsson, 2011). Estrogen deficiency can affect physiological functions and cause osteoporosis, lipid abnormalities, atherosclerosis, and obesity (Dumont et al., 2015; Pollanen et al., 2011). It should be noted, however, these results are in contrast with those of the studies on high circulating estrogen levels, a risk factor for postmenopausal breast cancer (Boyapati et al., 2004; Lukanova et al., 2004), showing that estrone, estradiol, and free estradiol are associated with increased body mass index. The main source of circulating estrogens in postmenopausal women are derived from androgen aromatization in the peripheral tissues such as notably adipose tissue (Feigelson et al., 2004).
A decline in estrogen levels in menopausal women is associated with the loss of muscle mass and function; therefore, it has been suggested that menopause can accelerate sarcopenia (Messier et al., 2011). In a human study, van Geel et al. (2009) reported that estrogen levels were positively associated with a lean body mass in menopausal women. Moreover, several studies have shown that estrogen replacement therapy attenuates age-related decline in muscle mass and size in postmenopausal women (Ronkainen et al., 2009; Taaffe et al., 2005). In addition, a decrease in muscle strength is also an important indicator of sarcopenia, which results from the loss of estrogen. Some studies have shown that loss of muscle strength is related to estrogen deficit during menopause (Carville et al., 2006; Cooper et al., 2008; Kurina et al., 2004). Furthermore, Moran et al. (2006) suggested that the decrease in estrogen levels resulted in reduced force generation in the soleus muscle of OVX mice. In particular, it is well known that the decrease in muscle mass with aging is more strongly affected by the type II muscle than the type I muscle (Verdijk et al., 2007). Widrick et al. (2003) showed that the type II muscle was smaller than the type I muscle in menopausal women regardless of HRT use. In addition, the ERs in muscles are expressed more specifically in the type II muscle (Brown, 2008).
Menopause status is not a major contributor to the loss of muscle mass in healthy women aged 18 to 85 years, although the loss of muscle mass is positively correlated with age (Tanko et al., 2002). Maltais et al. (2009) demonstrated that low levels of estrogen seem to be associated with a decline in muscle mass and strength; however, the conflicting results of these studies make it difficult to confirm this relationship. Therefore, it remains unclear whether the loss of estrogen during menopause induces a decline in muscle mass, function, and muscle fiber type changes. Further studies are needed to identify the mechanisms underlying skeletal muscle loss and muscle fiber type changes caused by menopause-induced estrogen deficiency.
It is well known that the balance between protein synthesis and protein degradation determines the maintenance of muscle quality, among which Akt/mammalian target of rapamycin (mTOR) signaling is an important regulator that may impact muscle quantity and quality (Shi et al., 2019). Protein synthesis is enhanced by the phosphorylation of p70S6K and 4E-binding protein 1, which activate mTOR via Akt (Bodine et al., 2001). In addition, Akt is reported to decrease the activity of forkhead box protein O (FOXO) transcription factors, which are associated with protein degradation (Sandri et al., 2004). Previous studies have demonstrated that estrogen deficiency in OVX rats induces a decrease in muscle Akt phosphorylation, which causes a decrease in muscle mass (Sitnick et al., 2006). Dieli-Conwright et al. (2009) suggested that HRT suppresses the expression of the transcription factor FOXO3A, a skeletal muscle atrophy gene. In addition, estrogen supplementation alleviates sepsis-induced skeletal muscle atrophy by the expression of atrogin-1 and MuRF1 downstream of FOXO1 (Zhao et al., 2017). These studies demonstrate that estrogen may inhibit protein degradation by regulating Akt/mTOR signaling, FOXO, and its downstream signals, all of which play an important role in delaying skeletal muscle loss.
ESTROGEN AND MITOCHONDRIA IN THE SKELETAL MUSCLE
Estrogen has three ERs that mediate its effects. There are two different forms of ERs: (a) ERα and ERβ, nuclear ERs that are members of the family of ligand-activated transcription factors, and (b) G-protein coupled ER, which is a membrane ER activated by estradiol. Growing evidence suggests that ERs are present in skeletal muscles (Lemoine et al., 2003) and in the nuclei of muscle fibers in the form of ERα and ERβ (Wiik et al., 2009). Therefore, it is not surprising that estrogen affects mitochondria in skeletal muscle.
Menopause and ovariectomy induce alterations in muscle biology and function. Skeletal muscle in OVX mice showed a decrease in the use of fatty acid substrates, reduced mitochondrial content, and decreased expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, which could be reversed by treatment with the major circulating estrogen 17β-estradiol (E2) (Cavalcanti-de-Albuquerque et al., 2014). Nagai et al. (2016) reported that OVX mice showed reduced resistance to fatigue, which is known to depend on mitochondrial mass. In addition, muscle-specific ERα knockout mice showed decreased muscle mass, induced obesity, insulin resistance, muscle mitochondrial dysfunction, impaired mitochondrial dynamics, and diminished mitophagy (Ribas et al., 2016). Furthermore, previous studies have demonstrated that E2 plays a major role in the inhibition of mitochondria-mediated apoptosis (Boland et al., 2008; Vasconsuelo et al., 2011).
Since estrogen deficiency in skeletal muscles induces the reduction of both mitochondrial biogenesis and dysfunction, it is assumed that mitochondrial dynamics, mitophagy, and mitochondria-mediated apoptosis would also be impaired by estrogen deficiency. A recent study suggested that the protein levels of mitochondrial dynamics (fusion) markers, including Mfn1, Mfn2, and Opa1, were decreased in the white gastrocnemius muscle of OVX rats (Capllonch-Amer et al., 2014). In addition, the protein level of cytochrome c, a mitochondria-mediated apoptosis marker, has been shown to be high in skeletal muscles of mice with E2 deficiency (Karvinen et al., 2021). However, the underlying mechanisms of ovariectomy-induced mitochondrial dysfunction should be elucidated through additional research.
EFFECTS OF EXERCISE ON THE SKELETAL MUSCLE IN ESTROGEN DEFICIENCY
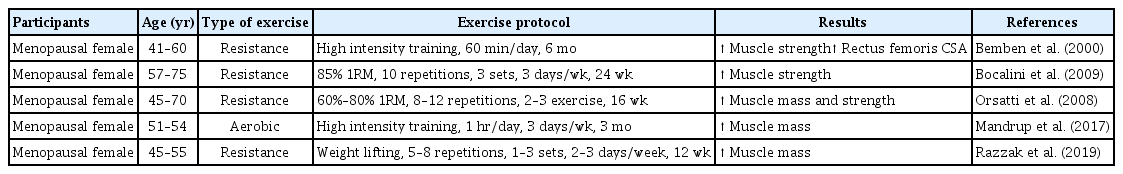
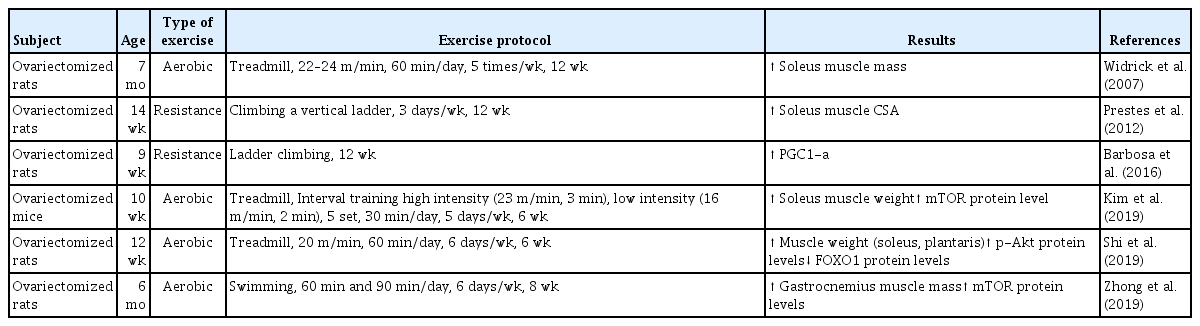
HRT is widely used as a therapeutic intervention to treat menopause; however, there are many risks associated with this therapy in humans, such as in endometrial and breast cancer patients (Olson et al., 2007; Stefanick et al., 2006). Conversely, exercise training is well characterized as an important contributor to muscle mass and strength and is considered to have a therapeutic effect on age-related sarcopenia. Recent systemic reviews and meta-analyses have found that exercise training has positive effects on muscle strength and physical performance in older adults (Bao et al., 2020; Escriche-Escuder et al., 2021; Wu et al., 2020). Furthermore, Chen et al. (2017) demonstrated that aerobic, resistance, and combined exercise training improved skeletal muscle mass in the elderly populations with sarcopenic obesity after training interventions. Taken together, regular exercise training may improve quality of life after menopause, with changes in the skeletal muscle (Elavsky and McAuley, 2005). Therefore, physical exercise, including aerobic and resistance exercise training, is recommended as a nonpharmacological intervention for menopausal treatment. Numerous human and rodent studies have demonstrated the beneficial effects of exercise training on estrogen deficiency-induced skeletal muscle impairment (Tables 1, 2).
Effect of aerobic exercise training on skeletal muscle in estrogen deficiency
It is well known that exercise training improves body composition, muscle strength, bone mineral density, and cardiorespiratory capacity (Elavsky and McAuley, 2009). Aerobic exercise training improves aerobic capacity, cardiovascular function, and metabolic regulation (Konopka and Harber, 2014). In postmenopausal women, aerobic exercise training improves metabolic and cardiovascular disease conditions (Manson et al., 2002). Zhong et al. (2019) revealed that swimming significantly increased gastrocnemius muscle mass in OVX rat. Moreover, aerobic exercise training for 12 weeks increased soleus muscle mass by 11% in OVX rats (Widrick et al., 2007). A recent study showed that 6 weeks of treadmill exercise training increased the muscle mass of the plantaris and soleus muscles in OVX mice (Shi et al., 2019). Collectively, aerobic exercise training has positive effects on skeletal muscle mass in estrogen-deficient conditions.
Effects of resistance exercise training on the skeletal muscle in estrogen deficiency
Resistance exercise training is associated with increased muscle strength, muscle mass, and bone mass (Barry and Carson, 2004). Several studies have demonstrated that progressive resistance training can change body composition, reverse sarcopenia, and recover the aggravation of muscle structure associated with menopause (Bocalini et al., 2009; Fjeldstad et al., 2009; Orsatti et al., 2008). Orsatti et al. (2008) revealed that muscle mass was significantly increased after 16 weeks of resistance exercise training in postmenopausal women. In addition, Tracy et al. (1999) observed a significant increase in the cross-sectional area of the quadriceps muscle after 9 weeks of resistance exercise training in postmenopausal women (Tracy et al., 1999). Another study in postmenopausal women showed that lean body mass increased and fat tissue decreased after 12 months of resistance exercise training (Teixeira et al., 2003). Thus, resistance exercise training plays an important role in the muscular function and quality of life in postmenopausal women.
CONCLUSIONS
Estrogen deficiency accelerates age-related sarcopenia via mitochondrial dysfunction. In particular, mitochondrial dynamics, mitophagy, and mitochondria-mediated apoptosis are believed to be associated with estrogen deficiency-induced sarcopenia. Resistance exercise training as a therapeutic intervention could be an important strategy for menopausal women to improve the muscle strength that determines sarcopenia, while a more structured program for aerobic exercise training may be required in some cases. Although there have been conflicting results regarding the effects of exercise training on the skeletal muscle induced by estrogen deficiency, many studies have shown that aerobic and resistance exercise training has positive effects on skeletal muscle mass, strength, and estrogen levels in menopausal women and OVX rodents. Further studies are needed to elucidate the optimal exercise protocols (e.g., exercise type, intensity, volume, etc.), which improve skeletal muscle mass and function, especially in postmenopausal women, and the cellular and molecular mechanisms underlying the beneficial effects of exercise training on the skeletal muscle against estrogen deficiency-related sarcopenia.
ACKNOWLEDGMENTS
This work was supported by Inha University Research Grant.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.