The influence of mat Pilates training adherence on strength and flexibility parameters in breast cancer survivors undergoing hormone therapy
Article information
Abstract
The aim of this study was to evaluate the influence of adherence to mat Pilates training on lower and upper body strength and flexibility in breast cancer survivors. Breast cancer survivors (≥40 years) with cancer stages 0–III undergoing hormone therapy participated in this study. For this secondary investigation only the intervention group was analyzed, divided into low and high training adherence. Participants performed a 60-min session of mat Pilates, 3 times/wk, for 24 weeks. Concentric, eccentric, and isometric hip flexor-extensor muscle peak torque, and isometric maximal strength parameters of the shoulder abductors, trunk extensors, and handgrip were assessed. Physical activity level was analyzed as a control variable. The results showed that high training adherence improved (P<0.05) left shoulder abductor strength parameters and lower and upper body flexibility compared to baseline. The low training adherence group improved (P<0.05) trunk extensors, right and left shoulder abductor strength parameters, handgrip strength, and extensor-flexor peak torque compared to baseline. There were no differences (P>0.05) between high and low adherence for physical activity level before and after the intervention. Therefore, it appears that higher training adherence most influences some strength parameters and flexibility, while fewer sessions enable the achievement of significant results for shoulder abductor and hip extensor-flexor muscle strength parameters.
INTRODUCTION
The incidence of breast cancer is growing worldwide, which appears to be influenced by unhealthy habits (i.e., sedentary behavior, improper diet, among others) (Bray et al., 2018). Nonetheless, early detection and treatments have increased life expectancy among breast cancer survivors (Lee Mortensen et al., 2018). When addressing hormone therapy, whether aromatase inhibitors (AIs) or selective modulators of estrogen receptors (SMREs), different adverse effects are observed (Early Breast Cancer Trialists’ Collaborative Group, 2015). In this sense AIs are related to moderate to severe joint stiffness and pain, while breast cancer survivors undergoing SMREs reported fatigue, and lower and upper body numbness, which could influence muscle force production, affecting physical functional capacity (Bray et al., 2018). Breast cancer surgery can also lead to side effects, such as decreasing shoulder range of motion and loss of upper body strength (Gho et al., 2013).
Considering the side effects of breast cancer treatments, studies have proven the beneficial effects of different exercise models on lower body muscle power and strength (Santagnello et al., 2020), physical functional capacity, and shoulder range of motion (Zengin Alpozgen et al., 2017). Despite these beneficial effects in mitigating the side effects of breast cancer treatments (Hardee et al., 2014), breast cancer survivors spend more time in sedentary behavior and in light physical activity intensity. Moreover, only 10%–15% of this population meet the minimum recommendations for exercise per week (150 min of moderate intensity or 75 min of vigorous intensity) (Jones et al., 2020). According to Jones et al. (2020), treatment-related barriers are challenging for exercise interventions.
In this scenario, the Pilates method, a mind-body exercise connecting breathing with concentration during each movement, could be suitable for breast cancer survivors, since it is safe and is widely performed among women (Mikalački et al., 2017). Among the many benefits of the Pilates method, we highlight joint mobility, muscle flexibility, strength, and balance, following fundamental principles (i.e., breathing technique, concentration, control, core stabilization, movement quality and flow) (Latey, 2002). However, these previously highlighted benefits can be compromised due to the low adherence of breast cancer survivors to the training program, which is associated with psychological, sociodemographic, and clinical factors (Kampshoff et al., 2016).
According to Fairman et al. (2020), adherence to an exercise program is defined by the degree of attendance, in other words, the number of sessions a participant participates in. In this context, to date there are no studies about the influence of adherence to mat Pilates training on physical performance demands in breast cancer survivors undergoing hormone therapy (AIs and SMREs). Therefore, as a secondary analysis of this investigation (Bertoli et al., 2020; Bertoli et al., 2022a), the aim of this study was to analyze the influence of adherence to mat Pilates training on lower and upper body muscle strength and flexibility in breast cancer survivors undergoing hormone therapy. It was hypothesized that breast cancer survivors with higher training adherence would obtain better results than those with lower attendance.
MATERIALS AND METHODS
Study design
The HAPiMat study (acronym translated to English as Health and Action Mat Pilates from Portuguese Ação e Saúde Pilates de Solo) is a 24-week randomized controlled clinical trial with breast cancer survivors undergoing AIs or SMREs, which was approved by the Sao Paulo State University Ethics Committee under the protocol CAAE 01993118.1.0000.5402 according to the Helsinki Declaration. The Brazilian Clinical Trials Registry approved this study under protocol RBR-3253dz. The participant recruitment started in February of 2019. The research protocol was published as a preprint with open access (Bertoli et al., 2020).
Participants
This study was disclosed through social media and flyers in the Regional Cancer Hospital. Breast cancer survivors undergoing hormone therapy who had previously participated in research studies of our laboratory were also invited to join in. When the individuals contacted the laboratory through telephone, the investigation procedures were explained and they were invited to participate. Thereafter, the participants signed the written inform consent on the first visit to the laboratory. A total of 1,100 registered breast cancer survivors were found. Due to the inclusion-exclusion criteria, 43 breast cancer survivors were eligible and randomized into control group n=22 and intervention group n=21. Randomization was carried out using the Excel program by persons not involved in the research. The participants were stratified by hormone therapy type. For this secondary analysis, only the intervention group was used for analysis (Fig. 1).
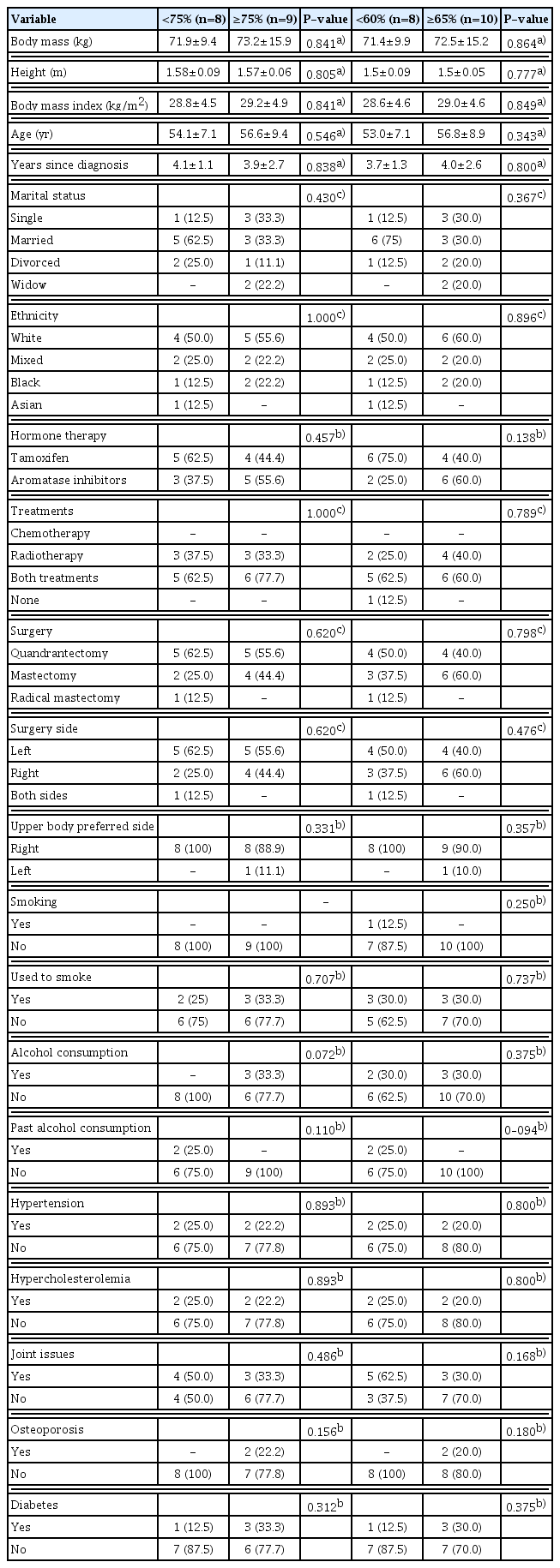
The sample size was carried out for 2 groups and 2 repeated measures, which resulted in 8 participants per group, taking into account an effect size of 0.8, alpha error <0.05, nonsphericity correction €=1, correlation between the repeated measures=0.5, and desired power (1-ß error)=0.80. G*Power (University of Düsseldorf, Dusseldorf, Germany) was used for analysis. For adherence to Pilates training, <75% and ≥75% attendance was considered for lower body strength parameters and flexibility collected until the end of the intervention (24 weeks), and for shoulder abductor and trunk extensor muscle strength parameters, adherence to training <60% and ≥60% attendance was considered, collected immediately after 12 weeks due to mechanical problems with the equipment.
The difference in percentages of attendance used was due to the decision to have a similar number of participants in each low and high adherence group. Comparisons between number of sessions and percentages according to low and high adherence groups are presented in Table 1. For the per protocol analysis carried out in the secondary analysis, participants were excluded if they began any type of systematic training during the intervention or stopped hormone therapy.

Mean and standard deviation of participants’ number of sessions and percentage attended according to low (<75%) and high adherences (≥75%) when considered 24 weeks of interventions, and low (<60%) and high adherences (≥60%) for 12 weeks of intervention
Participants were required to be ≥40 years of age, with confirmed breast cancer stages 0–III (Pourrahmat et al., 2021) certified by a physician and stated in the medical record, be undergoing hormone therapy, have clearance from their physician to practice mat Pilates and physical tests, live in or near the city where the research was carried out, not be currently practicing Pilates or any other regular resistance training, and sign the consent form.
Mat Pilates program
The intervention was held 3 times a week (60 min per session) by a qualified mat Pilates instructor and an undergraduate strength and conditioning professional, who helped to better control each session (2:7). The difficulty and number of exercise repetitions were increased over the weeks, starting from beginner, followed by intermediate, and ending with advanced level (for some of the exercises). In cases where the participant was not able to progress, she continued in the same level of exercise. The mat Pilates general planning and exercises (Table 2) were based on the Bertoli et al. (2020, 2022a) study, which focused on exercise load progression according to the participants’ characteristics. This protocol is appropriate for breast cancer survivors since the exercise focuses on joint mobility, muscle strength, and flexibility, which are compromised in the short and long-term in this population due to breast cancer treatments, such as breast cancer surgery, radiotherapy, chemotherapy, and hormone therapy. Furthermore, the exercise complexity can be adapted to each participants’ capacity (Wells et al., 2012).
Safety
The limitations of the participants to perform the exercises were respected by adapting the exercises. Participants were exempted from performing the physical tests if they had a pre-existing musculoskeletal condition that could cause harm. Adverse events (i.e., muscle and joint pain, muscle soreness, dizziness) that occurred during the assessments and intervention were recorded.
Upper body resistance test
Upper body strength parameters were evaluated until week 12 due to mechanical problems with the equipment. The same protocol used in Bertoli et al. (2022b) was also carried out in the current study. Three maximal isometric voluntary contractions of 5 sec with a rest period of 90 sec were performed with a load cell (Cefise n2000 pro 2.0, Nova Odessa, SP, Brazil) in the following muscle groups: (a) trunk extensors, (b) right and left shoulder abductors. For the trunk extensor muscles, participants were required to remain in the standing position, with the knee and hip flexed at 120°, the back and elbow in the extended position, and the trunk extended. The shoulder abductor muscles were assessed in a standing position at 65° shoulder abduction. The participants were instructed to perform the contractions as fast and hard as possible. A fourth maximal isometric voluntary contraction was performed in case of a 5% coefficient of variation between the attempts. The highest maximal isometric voluntary contraction was considered for analysis.
To compare the upper body resistance parameters, the time to achieve maximal force (TFmax), maximal force (Fmax) normalized by body mass (Fmax/kg) was evaluated. To obtain muscle power, the absolute rapid force index (RFI) was assessed by dividing absolute Fmax by the TFmax (Fmax/t). The Fmax/kg was divided by TFmax to obtain the normalized RFI/kg (Bertoli et al., 2022b).
Lower body resistance tests
Before testing, participants completed a warm up of 5 min of cycling at 50 W on a cycle ergometer (Ergo-fit 167 Cycle, Pirmasens, Germany). Hip flexor and extensor muscles were assessed with an isokinetic dynamometer (BIODEX Medical Systems 4, Shirley, NY, USA). The same protocol as adopted in Bertoli et al. (2022a) was used to position the participants. Once the participant was positioned in the dynamometer, a warm up was performed with ten repetitions of the flexor-extensor muscle concentric contractions at 120°/sec.
Subsequently, the isometric tests consisted of three contractions of 5 sec and a fourth repetition was performed when the coefficient of variation was higher than 5%. The maximal isometric voluntary contraction for the flexor muscles was performed at 15° of hip flexion, while for the extensor muscles, contractions were performed at 100° (Bertoli et al., 2019). After 2 min of rest, the isokinetic tests were performed at concentric/eccentric contractions for the flexor muscles at 30° range of motion. For the extensor muscles, eccentric/concentric contractions were performed at 70° range of motion. The angular velocity for both muscle groups was 60°/sec with 2 min of rest between sets (2 sets). Peak Torque and mechanical work were normalized by the participant’s body mass (Nm/kg and J/kg, respectively).
Maximum isometric handgrip
The maximal isometric voluntary contraction of the forearm muscle was assessed with an adjustable hand dynamometer (Standard Cefise dynamometer, Nova Odessa, SP, Brazil) in the right and left hands. Participants remained in the sitting position with the back straight, and the hip and elbow flexed at 90° (Bohannon et al., 2006). Three intercalate contractions of each hand were performed until the participants achieved their maximal isometric voluntary contraction with a 2-min rest between attempts. The best result was used for analysis. The result was normalized by the participant’s body mass (kgf/kg) (Bertoli et al., 2022b).
Flexibility
The flexibility of the lumbar and hip joints was assessed with the sit-and-reach test, while shoulder and triceps flexibility were evaluated with the back-scratch test. Both right and left lower and upper body were analyzed. Two repetitions were performed on each side and the best one was used for analysis. Both the modified sit-and-reach and back-scratch tests were evaluated according to the Rikli and Jones (2013) protocol.
Physical activity level
This variable was only obtained pre and post 24 weeks of intervention. The physical activity level was measured with Actigraph triaxial accelerometers, model GT3X (Actigraph LLC, Pensacola, FL, USA). The device measures and records acceleration variations with magnitudes covering approximately 0.05 to 2.5 g (g= 9.8 m/sec2) within a frequency range of 0.25 to 2.5 Hz. Each data sample (counts) was summed over a particular time interval (epoch, of 10 sec). The participants were instructed to wear the device at the level of the waist for five weekdays and two weekend days. Participants were advised to wear the accelerometer during daytime in the waking hours, and only remove it when performing water activities, personal hygiene, and while sleeping (Rossi et al., 2017).
Data analysis was carried out with specific software (ActiLife6 - data analysis software by Actigraph LLC). The raw accelerometer data were translated into minutes of physical activity and the intensity was analyzed. Full days of monitoring with at least 10 hr of use (600 min of recording) were used for analysis (Migueles et al., 2017), with at least 4 days of monitoring (3 weekdays and 1 weekend day, excluding the sleeping hours) (Trost et al., 2005). The following cut points were considered: moderate physical activity between 1,952 and 5,724 (3.00–5.99 metabolic equivalents [METs]); vigorous physical activity between 5,725 and 9,498 (6.00–8.99 METs); and very vigorous physical activity comprised values greater than 9,499 counts per minute (9 METs) (Freedson et al., 1998). The habitual physical activity was expressed in minutes of moderate, moderate+vigorous+very vigorous physical activity, and counts per minute (the sum of counts of the three accelerometer axes divided by the time used) (Freedson et al., 1998).
Statistical analysis
Descriptive analysis was carried out. Baseline characteristics of the participants were compared between low and high adherence with the Student t-test, chi-square, and Fisher exact test. The generalized estimating equations (GEEs) model was used to compare each outcome measure. The adherence analysis was performed only for those participants assigned to a treatment who actually received, complied with, and completed the intervention, “per protocol analysis.” The Bonferroni post hoc test was applied when significant differences were identified for the time. The effect size was calculated based on differences in groups with unequal sample sizes within a pre-post-control design, and classified according to the Cohen d statistic, into <0.0 (adverse effect), 0.0–0.1 (no effect), 0.2–0.4 (small effect), 0.5–0.7 (intermediate effect), and ≥0.8 (large effect). The delta percentage (Δ%) was calculated based on low and high adherence of each participant: [(postintervention–preintervention)/preintervention]*100, thereafter, the Δ% mean was calculated. Independence chi-square was applied to verify the association between hormone therapy versus adherence (low and high) with correction by the Fisher exact test. IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. Statistical differences were considered when P<0.05.
RESULTS
Baseline
There were no statistical differences between low and high adherence at baseline for body weight, height, body mass index, age, and sociodemographic variables (Table 3). None of the baseline analyses for any of the primary and secondary outcomes between both high and low adherence groups presented statistical differences (P>0.05). The same was observed post intervention between groups (Figs. 2–4).
Mean and standard deviation of participants’ characteristics and health aspects compared between low (<75%) and high adherences (≥75%) when considered 24 weeks of interventions, and low (<60%) and high adherences (≥60%) for 12 weeks of intervention
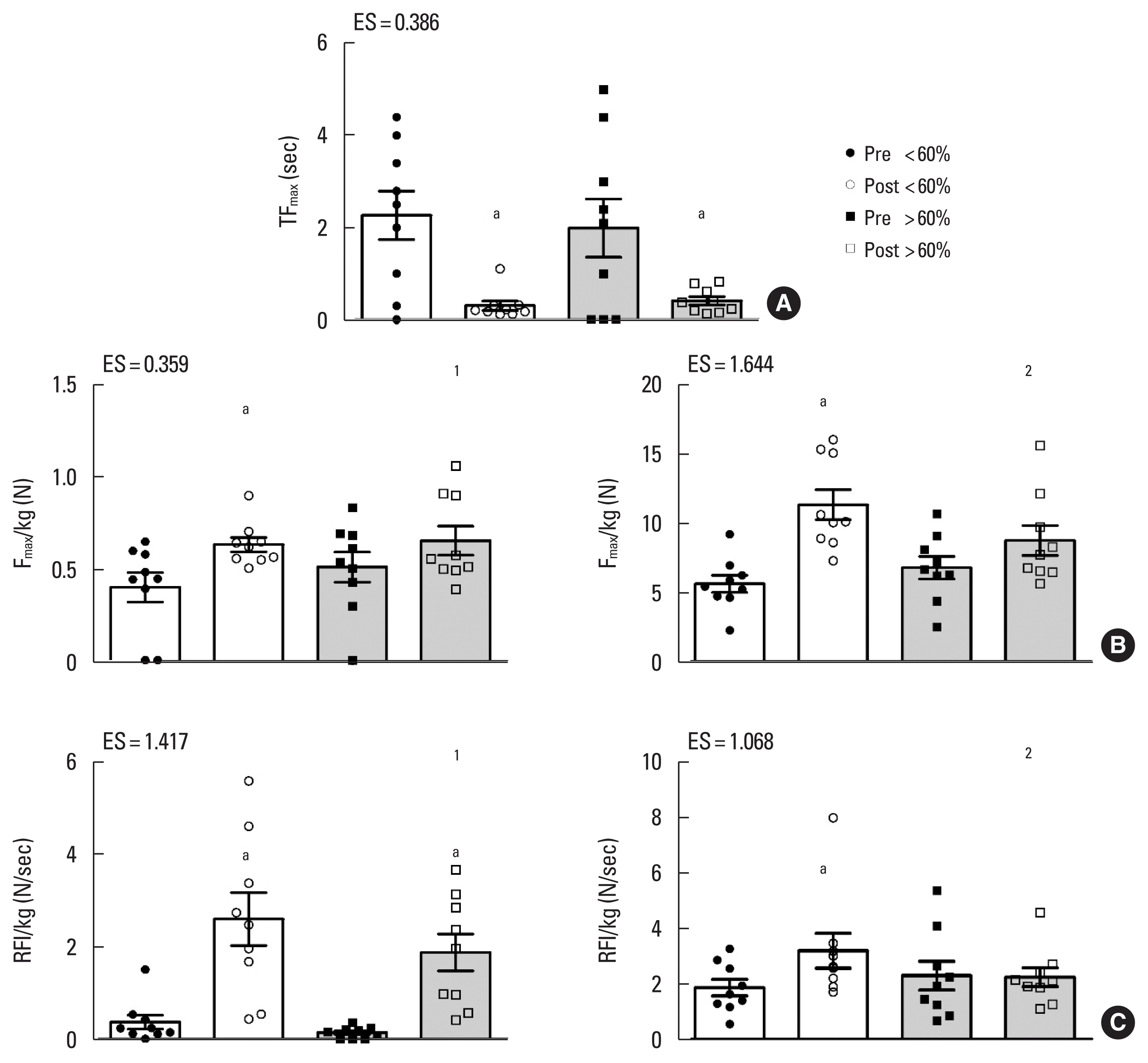
Upper body strength parameters adherence analysis. (A) Left shoulder abductors. (B.1) Right shoulder abductors. (B.2) Trunk extensors. (C.1) Left shoulder abductors. (C.2) Trunk extensor muscles adherence analysis. TFmax, time to achieve maximal force; Fmax, maximal force; RFI, rapid force index; Pre, preintervention; Post, postintervention; ES, effect size. Letter a indicates significant difference within time after Bonferroni post hoc comparison (P<0.05). Effect size d<0.0: adverse effect. Effect size d=0.0–0.1: no effect. Effect size d=0.2–0.4: small effect. Effect size d=0.5–0.7: intermediate effect. Effect size d≥0.8: large effect.
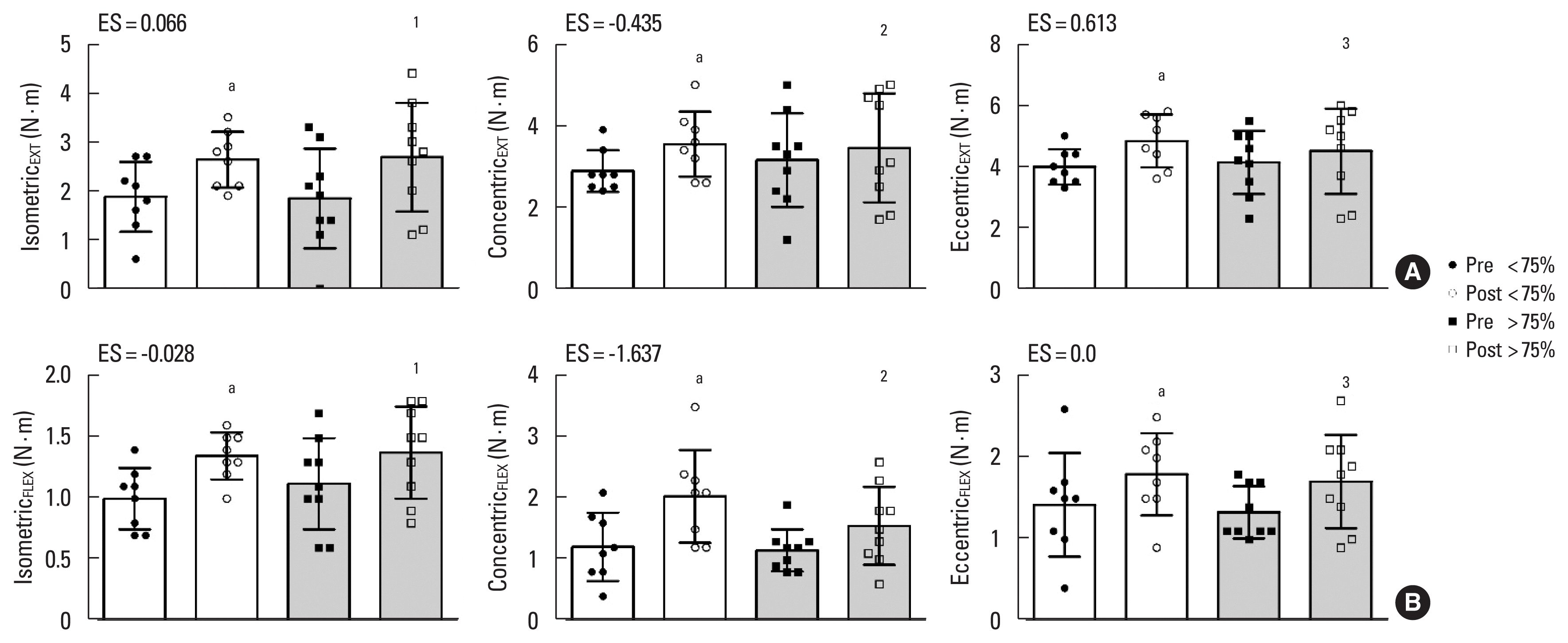
Extensor-flexor muscles hip torque adherence analysis. (A.1) Isometric extensor muscles peak torque (PT Extiso). (A.2) Concentric extensor muscles peak (PT Extcon). (A.3) Eccentric extensor muscles peak (PT Extecc). (B.1) Isometric flexor muscles peak torque (PT ExtFLEX). (B.2) Concentric flexor muscles peak (PT Flexcon). (B.3) Eccentric flexor muscles peak (PT Flexecc). Pre, preintervention; Post, postintervention; N.m, Newton times meters; ES, effect size. Letter a indicates significant difference within time after Bonferroni post hoc comparison (P<0.05). Effect size d<0.0: adverse effect. Effect size d=0.0–0.1: no effect. Effect size d=0.2–0.4: small effect. Effect size d=0.5–0.7: intermediate effect. Effect size d≥0.8: large effect.

HGS, lower and upper body flexibility adherence analysis. (A) Left HGS. (B.1) Right upper body flexibility. (B.2) Left upper body flexibility. (C.1) Right lower body flexibility. (C.2) Left lower body flexibility adherence analysis. HGS, handgrip strength; Pre, preintervention; Post, postintervention; ES, effect size. Letter a indicates significant difference within time after Bonferroni post hoc comparison (P<0.05). Effect size d<0.0: adverse effect. Effect size d=0.0–0.1: no effect. Effect size d=0.2–0.4: small effect. Effect size d=0.5–0.7: intermediate effect. Effect size d≥0.8: large effect.
Upper body strength parameters
Left shoulder abductor TFmax improved (P<0.001) after the intervention for both <60% and ≥60% adherence compared to baseline (Δ% 148; −1,813 respectively) (Fig. 2A). Right shoulder abductor (Δ% 1,627) and trunk extensor muscles (Δ% 139) Fmax/kg improved after the intervention (P=0.006, P<0.001, respectively) for <60% adherence (Fig. 2B.1, B.2). Left shoulder abductor musclesRFI/kg increased (P<0.001) after the intervention for both <60% and ≥60% adherence (Δ% 4,383 respectively) (Fig. 2C.1), however, trunk extensor muscle RFI/kg improved (P= 0.042) after the intervention for <60% adherence (Δ% 104), (Fig. 2C.2).
Lower body strength parameters
The hip extensor (Δ% 60) and flexor (Δ% 271) muscle isometric peak torque showed significant increments (P=0.012, P=0.002, respectively) after the intervention compared to baseline for <75% adherence (Fig. 3A.1, B.2). The same was observed for the extensors concentric (Δ% 23), and eccentric (Δ% 22) peak torque (P= 0.036, P=0.014, respectively) (Fig. 3A.1), and for the flexors concentric (Δ% 105.9) and eccentric (Δ% 61.7) peak torque (P=0.006, P=0.006, respectively) (Fig. 3B.1).
Maximum isometric handgrip
Left handgrip strength showed significant improvements (P= 0.033) after the intervention compared to baseline or <75% adherence (Δ% 17.9) (Fig. 4A).
Lower body flexibility
Left upper body flexibility (Δ% −21,322) (Fig. 4B.2), and right (Δ% 48,958) and left (Δ% 27,524) lower body flexibility (Fig. 4C.1, C.2) showed improvements (P=0.001, P=0.003, P=0.001, respectively) after the intervention compared to baseline for the ≥75% adherence.
There were no significant differences (P<0.05) between pre and post intervention according to training adherence for the following variables: (a) right handgrip strength and upper body flexibility after 24 weeks of intervention; (b) trunk and right shoulder abductor TFmax, left shoulder abductor Fmax, and right shoulder abductor muscle RFI after 12 weeks of intervention.
Physical activity level
It can be observed that the low adherence group increased average moderate and vigorous physical activity/day (negative large effect size d=−1.329), moderate/day (intermediate effect d=0.609), and steps/day after 24 weeks of intervention (negative intermediate effect d=−0.609), however without significant differences between pre and post 24 weeks of intervention between low and high adherence (<75% and ≥75% respectively) (Table 4).
Association between hormone therapy vs. adherence
No significant association between hormone therapy and adherence was found during the first half of training (12 sessions; χ2(1)= 2.20, P>0.05; φ=0.35), or for the total sessions (24 sessions; χ2(1)=0.55, P>0.05; φ=0.181).
DISCUSSION
The aim of this study was to analyze the influence of mat Pilates training adherence on muscle strength and flexibility, which was a secondary analysis of the HAPiMat study (Bertoli et al., 2020; Bertoli et al., 2022a). The results showed that the group with lower training adherence presented increased hip torque variables and left handgrip strength, while the group with higher training adherence presented improved left shoulder abductor TFmax and RFI, and left and right upper and lower body flexibility after 24 weeks of intervention. On the other hand, Fmax (right shoulder abductor and trunk extensor muscles) and trunk extensor muscles RFI increased in the lower adherence group after 12 weeks of intervention. There were no differences between groups with high and low attendance after the intervention assessment. Therefore, the hypothesis of this study was unfulfilled for most of the upper and lower body strength parameters, except for upper and lower body flexibility, and left shoulder abductor TFmax and RFI variables.
According to Kampshoff et al. (2016), participant adherence to exercise reflects the attendance in the programmed sessions and the commitment to the prescribed exercise, thus, it is expected that the positive results of exercise intervention programs depend on participants’ adherence and commitment. This tendency was observed for the upper and lower body flexibility, and left shoulder abductor TFmax and RFI. Nonetheless, the majority of the results of the upper and lower body strength parameters assessed in this investigation are contrary to what was expected, which is that higher adherence would induce greater improvements. Recently, a meta-analysis suggested that higher adherence rates to a supervised resistant training program could also result in greater adaptations owing to an increase in training volume, specifically in strength parameters (Lacroix et al., 2017).
In this scenario, the physical activity level was analyzed through moderate + vigorous physical activity, moderate physical activity level, and steps/day, which showed no differences between pre and post intervention between and within low and high adherence groups (<75% and ≥75%, <60% and ≥60% of attendance respectively). Nonetheless, the mean of each variable was higher for the low adherence group with a large negative, positive intermediate, and negative intermediate effect size for moderate+vigorous physical activity level, moderate physical activity level, and steps/day respectively. From these results, it could be hypothesized that these outcomes might be related to the fact that the low adherence group presented increased strength parameters mainly of the lower body.
In the current study, higher adherence was defined as attending ≥75% of the prescribed sessions for the variables measured after 24 weeks of the intervention, while ≥60% was considered high adherence for the variables measured at the end of 12 weeks of the intervention due to mechanical issues with the equipment. With regard to the percentages of attendance in this investigation, there was a group of breast cancer survivors that might have faced some barriers to adherence (i.e., treatment side effects, psychological effects and self-efficacy, and lack of time) to the mat Pilates program, which is in agreement with the literature (Kampshoff et al., 2016). Therefore, maintaining high adherence in exercise interventions might be challenging for breast cancer survivors, especially for those undergoing treatment (Witlox et al., 2019). In this context, recent studies observed that most cancer survivors do not engage in physical exercise with a sufficient duration and intensity, spending most of their time in sedentary behavior (Boyle et al., 2016).
The fact that the lower adherence group obtained significant increments in strength parameters could be explained by the study of Mazzoni et al. (2020) with cancer patients undergoing treatment (including hormone therapy). Although the authors applied a motivation strategy, adherence was not optimal; nonetheless, when the participant attended the session, they were fully committed to the exercises. However, when addressing flexibility, which is recommended by the American College of Sports Medicine to be part of the cancer survivor training program (Schwartz et al., 2017), it was observed that higher adherence resulted in significant increases. From a breast cancer surgery perspective, increasing shoulder flexibility would reflect in better shoulder range of motion and mobility (Zengin Alpozgen et al., 2017), while from a hormone therapy standpoint, increasing not only upper but also lower body flexibility would mitigate some side effects (i.e., joint stiffness and pain) (Roberts et al., 2011). Moreover, flexibility tends to easily decline with the aging process, resulting in further physical functional capacity impairments (Zullo et al., 2020).
Conversely, the fact that in breast cancer survivors undergoing AIs and TMX, most of the strength parameters and flexibility improved after the mat Pilates intervention, might mitigate the treatment-related musculoskeletal side effects that may worsen over the years, such as, joint stiffness, arthralgia, muscle pain (Park et al., 2012), and handgrip strength decrement (Mazzoni et al., 2020). This factor occurs independently of adherence and commitment; it appears that for a minimal positive improvement response a few sessions are enough (range, 12–22 sessions, see details in Table 2).
This is the first study to compare the influence of mat Pilates training adherence on different muscle group strength and flexibility parameters in breast cancer survivors undergoing AIs and TMX. Nonetheless, some limitations must be acknowledged: (a) lack of blinding assessments, (b) not having a specific questionnaire to investigate possible barriers to attend the mat Pilates sessions, (c) not performing upper body assessments after 24 weeks of the intervention, and (d) a greater sample size would lead to stronger conclusions. In this scenario, and due to the relevance of exercise oncology, further research should be performed to compare high and low adherence of the Pilates Method on muscular strength and flexibility in different types of cancer survivors and at different stages of treatment.
In summary, participants with lower adherence (12 to 22 sessions) to the mat Pilates intervention presented significantly improved isometric, concentric, and eccentric hip extensor-flexor muscle peak torque after 24 weeks of intervention compared to baseline. The same was observed for handgrip strength. The high adherence group improved left shoulder abductor TFmax and RFI, as well as right and left upper and lower body flexibility after 24 weeks of intervention. Finally, the group with lower adherence improved left shoulder abductor TFmax, right shoulder abductor and trunk extensor Fmax, and left shoulder abductor and trunk extensor muscles RFI after 12 weeks of intervention.
ACKNOWLEDGMENTS
The authors thank the participants of this study, the Presidente Prudente Cancer Regional Hospital, the Health Professionals who gave their time and knowledge to the participants, and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), grant number 001 for the scholarship to J. Bertoli.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.



