Effect of 8-week high-intensity intermittent running exercise and weight training on muscle and DNA damage in male and female ski major college students
Article information
Abstract
This study assessed changes in blood muscle damage indicators and DNA damage indicators in lymph and urine after 8 weeks of high-intensity intermittent running and weight training in male and female college students majoring in skiing. This study aimed to find an effective training method by investigating differences in the effectiveness between men and women. A total of 20 male and female ski major college students conducted short-term high-intensity intermittent running and weight training in the morning and afternoon, respectively, 3 days a week for 8 weeks for 24 times in total. After 8 weeks of high-intensity intermittent running and weight training, changes in DNA damage indicators in the lymph and urine and muscle damage indicators in the blood were analyzed. The creatine kinase level significantly differed at rest pre-graded exercise testing (GXT) and 60 min of recovery post-GXT after training from that before training between the male and female groups. Although lactate dehydrogenase (LDH) levels decreased in both groups over time, no significant differences in LDH were found between the two groups. Second, DNA 8-hydroxydeoxyguanosine (8-OHdG) in the lymph was significantly different between the two groups at rest pre-GXT and 60 min of recovery post-GXT. 8-OHdG in the urine was significantly lower in the female group only at 60 min of recovery post-GXT. Partial sex differences were found in the reduction of muscle damage and DNA damage after 8 weeks of high-intensity intermittent running and weight training.
INTRODUCTION
Intermittent exercise, involving repetitive high-intensity full-body exercise within a short period, is a highly efficient exercise that achieves maximum heart rate (HRmax) and energy consumption. The American College of Sports Medicine recommends exercising at 80%–95% of the HRmax for 5 sec to 8 min for high-intensity intermittent exercise (HIIE) and performing low-intensity exercise during rest for the same amount of time as HIIE at 40%–50% of the HRmax. HIIE has been reported to have various effects compared with other exercises of different intensities; however, muscle damage after HIIE increases enzyme concentrations in the blood in general individuals and athletes (Lippi et al., 2008). Specifically, 1-time exercise induces muscle fatigue, increasing muscle-damaging materials. By contrast, long-term regular training reduces muscle-damaging materials for the same exercise load (Du and Sim, 2021).
Creatine kinase (CK) and lactate dehydrogenase (LDH), which have been proposed as indicators of muscle damage in the blood, are closely related to the intensity, duration, and amount of exercise and show various patterns depending on the fitness level of the body. An increasing interest has focused on exercise-induced DNA damage (Cakatay et al., 2010). High-intensity exercise can increase the oxygen intake of skeletal muscles by 100- to 200-fold, and such an excessive increase in oxygen intake overpowers the antioxidant system and produces lipid peroxidation. This can damage and deform DNA and proteins (Mastaloudis et al., 2004; Sen, 1995; Tanimura et al., 2008). Among DNA forms affected by oxidative damage, 8-hydroxydeoxyguanosine (8-OHdG) is a key oxidative nucleotide derivate of DNA helix damage. Studies have previously suggested that oxidative stress from physical exercise can cause DNA damage (Adelman et al., 1988; Levine, 1983).
The role of regular exercise in DNA damage is still unclear, and the relationship between regular exercise and DNA damage as an adaptation to HIIE, as it may be seen as a DNA damage repair process, should be investigated. On the contrary, various studies are actively investigating the factors that cause oxidative stress, DNA damage, and countermeasures. Some of those studies have suggested the potential role of sex in oxidative stress and DNA damage (Massafra et al., 2000; Viña et al., 2006).
Therefore, in this study, we aimed to investigate changes in muscle and DNA damage indicators after 8 weeks of HIIE in male and female ski major college students and assess the differences by sex.
MATERIALS AND METHODS
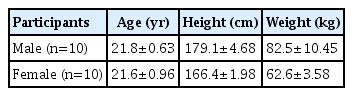
The study participants were randomly selected 20 college students, including 10 male and 10 female students, majoring in skiing at Hebei Sport University in Shijiazhung City, China. This study was approved by the Ethics Committee of a Tangshan Normal University (No: TSNU-2020-l01). The characteristics of the participants are shown in Table 1.
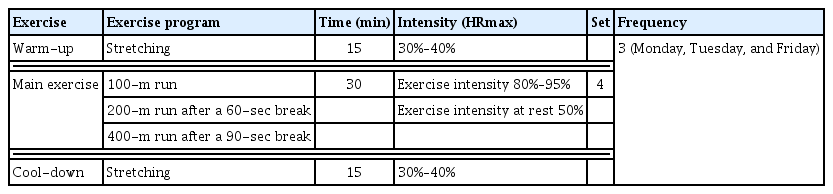
Running was considered a short-time HIIE and performed 3 times a week (Monday, Wednesday, and Friday) in the morning for 8 weeks for a total of 24 times. To maximize the exercise effects, weight training was performed in the afternoon. Running was conducted for 60 min per session, including 30 min of the main exercise and 15 min each for warm-up and cool-down. The running exercise was conducted at 80%–95% of the HRmax, and during rest, the participants ran at 50% of the HRmax. The exercise intervals were as follows: 100-m run, followed by 60 sec of rest, 200-m run, 90 sec of rest, and 400-m run. This was repeated 4 times, with 3-min rest between each set. The exercise intensity was basically done according to the guidelines of the American College of Sports Medicine, but HIIE was implemented by modifying the exercise program suggested by Du and Sim (2021) in consideration of the exercise ability that the subjects could perform (Table 2).
Weight training was conducted 80 min per session, including 60 min of the main exercise and 10 min each for warm-up and cool-down. A total of 13 different exercises were performed at 10–15 repetitions per set at 65%–75% of one-repetition maximum (1RM). Each exercise was conducted for 20–30 sec, and the rest time between each exercise was 20 sec. The rest time between upper body, abdominal, and lower body exercises was 60 sec, and the rest time between each set was 120 sec (Table 3).
Blood and urine samples of the participants were collected at 4-time points: at rest before the graded exercise test (GXT), immediately post-GXT, 60 min of recovery post-GXT, and at 120 min of recovery post-GXT before and after the 8-week-long HIIE running and weight training program. On the day of blood and urine collection, the Bruce protocol was conducted on a treadmill for GXT. This progressive overload exercise was conducted until all-out based on the rating of perceived exertion by Borg. The collected blood was centrifuged at 4°C at 3,000 rpm for 30 min at the Department of Diagnostic Testing. After centrifugation, the blood was stored in a freezer at 70°C. The urine was immediately frozen after collection and stored in a 75°C freezer until analysis. All variables were analyzed at the clinical laboratory and medical verification center of S hospital. CK and LDH levels were analyzed as muscle damage indicators. Changes in lymph and urine 8-OHdG levels were analyzed as DNA damage indicators.
In this study, all data analyses were conducted using the IBM SPSS Statistics ver. 26.0 (IBM Corp., Armonk, NY, USA). The mean and standard error were derived for each variable, and repeated measures analysis of variance was conducted to assess the effects of the variables on changes in muscle and DNA damage indicators between the groups. The significance level was set at 0.05.
RESULTS
Changes in LDH levels
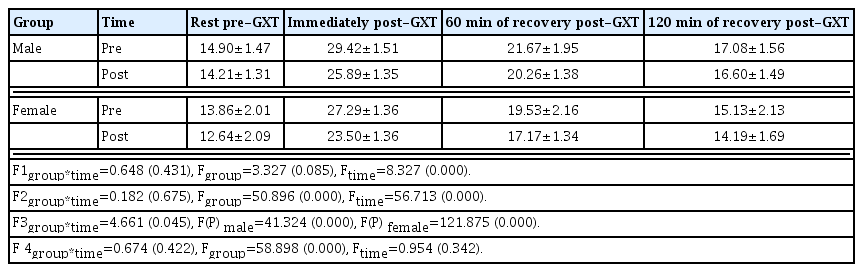
Changes in the LDH levels are presented in Table 4. No interaction was observed between the groups and time points at all 4-time point measurements.
Changes in CK levels
Changes in the CK levels are presented in Table 5. Interactions were found between the groups and time points at rest pre-GXT and 60 min of recovery post-GXT (P<0.05). The CK level significantly decreased at rest pre-GXT and 60 min of recovery post-GXT posttraining compared with that observed during pretraining in both male and female groups (P<0.001). By contrast, no interactions were found between the groups and time points immediately post-GXT and at 120 min of recovery post-GXT.
Changes in lymph DNA 8-OHdG levels
Changes in the lymph DNA 8-OHdG levels are presented in Table 6. Interactions were found between the groups and time points at rest before GXT and 60 min of recovery post-GTX (P< 0.05). The lymph DNA 8-OHdG level significantly decreased at rest pre-GXT posttraining than that observed at rest pre-GXT pretraining in both male and female groups (P<0.01). In the male group, the lymph 8-OHdG level significantly decreased at 60 min of recovery post-GXT posttraining compared with that observed at pretraining (P<0.01). However, no significant difference was noted between pre- and posttraining in the female group. By contrast, no interactions were observed between the groups and time points immediately post-GXT and at 120 min of recovery post-GXT.
Changes in urine DNA 8-OHdG levels
Changes in the urine DNA 8-OHdG levels are presented in Table 7. Significant interactions were noted between the groups and time points at 60 min of recovery post-GXT (P<0.05). In both male and female groups, urine DNA 8-OHdG levels posttraining were significantly decreased compared with those of pretraining (P<0.001). By contrast, no interactions were observed between the groups and time points at rest pre-GXT, immediately post-GXT, and at 120 min of recovery post-GXT.
DISCUSSION
Muscle damage following unfamiliar training or high-intensity exercises causes mechanical and oxidative stress. CK and LDH, which are used as indicators of muscle damage, are activated during high-intensity exercises and show different patterns depending on the fitness level of the body (Brancaccio et al., 2010; Deruisseau et al., 2004). Although various previous studies have reported that vigorous one-time exercise or high-intensity training induces muscle fatigue, increasing the concentration of muscle damage substances and causing structural damage to skeletal muscles (Bradley et al., 2010; Brancaccio et al., 2008; Nosaka et al., 2011), recent evidence suggests that regular training for a long period reduces muscle damage and muscle-damaging materials for the same exercise load (Du and Sim, 2021; Yang, 2015). Yang (2015) reported that CK levels significantly decreased in the exercise group after 6 weeks of high-intensity winter training in judo athletes. In a recent study, Du and Sim (2021) observed decreased CK and LDH levels after 8 weeks of interval training in sprinters.
Herein, after 8 weeks of HIIE, CK levels significantly decreased in both male and female groups at rest pre-GTX and 60 min of recovery post-GTX. Specifically, at rest pre-GTX, CK levels were more effectively reduced in the female group than in the male group, whereas at 60 min of recovery post-GTX, CK levels were significantly lower in the male group than in the female group. Although CK levels immediately post-GTX and at 120 min of recovery post-GTX decreased in both groups posttraining compared with that of pretraining, no significant differences were found in the CK levels between the two groups. By contrast, LDH levels decreased during posttraining compared with the levels during pretraining in both male and female groups at all 4-time point measurements; however, no significant differences were found in LDH levels between the two groups.
In agreement with the results of previous studies, our findings suggest that CK and LDH activity may vary depending on the body’s familiarity with high-intensity exercise and that CK and LDH activities decrease with long-term training. Although it is thought that increased physical strength leads to an improved defense mechanism against cellular damage, enzyme activity in the muscles and blood may vary depending on not only genetic factors, training method, exercise intensity, and exercise duration, but also individual characteristics of the participants.
Increased oxidative stress due to exercise can lead to oxidative modification of lipids, proteins, and nucleic acids, resulting in DNA damage (Cakatay et al., 2010; Sachdev and Davies, 2008). Specifically, unlike many previous studies reporting that high-intensity one-time exercise increases DNA damage (Mastaloudis et al., 2004; Tanimura et al., 2008; Tsai et al., 2001), regular training protects against exercise-induced DNA damage as an adaptive phenomenon involving the regulation of intracellular protein enzymes and repair enzyme system (Radák et al., 1999). Radák et al. (1999) reported that 8-OHdG levels significantly decreased in the group of 4-week-old mice that underwent swimming exercise for 14 months compared with the nonexercising group. In another study, Radák et al. (2000) also measured urine 8-OHdG levels in five well-trained super-marathon runners during a 4-day race. On day 1, 8-OHdG levels increased significantly, and on day 4, 8-OHdG levels decreased significantly compared with that measured in the first 3 days.
In our study, lymph 8-OHdG levels measured after 8 weeks of HIIE significantly decreased at rest pre-GTX in both male and female groups compared with that before training. Specifically, the 8-OHdG level was significantly lower in the female group than in the male group. On the contrary, at 60 min of recovery post-GTX, 8-OHdG levels significantly decreased in the male group, but not in the female group. 8-OHdG levels immediately post-GTX and at 120 min of recovery post-GTX decreased in both groups posttraining compared with that pretraining; however, no significant differences were found in 8-OHdG levels between the two groups.
Previous studies have suggested that sex can affect oxidative stress and that women may experience less oxidative stress than men (Goldfarb et al., 2007; Massafra et al., 2000; Viña et al., 2006). Although the effects were not related to training, Massafra et al. (2000) reported that estrogen, a female hormone, can increase superoxide dismutase (SOD) and glutathione peroxidase, thereby providing a protective mechanism against the generation of reactive oxygen species. In addition, Yamafuji et al. (1971) stated that steroids, male hormones, can induce DNA damage, leading to greater DNA damage in men than in women. Among studies supporting such theory with regard to oxidative stress and DNA damage during exercise, Tiidus (2000) reported that female rats had less exercise-induced oxidative stress than male rats after running until exhaustion. Furthermore, Sureda et al. (2008) observed that women had high mitochondrial SOD at rest and lower hydrogen peroxide levels after swimming at 75%–80% of the maximum capacity, suggesting that women have higher resistance to stress than men during high-intensity exercise.
The findings of this study showed that a decrease in the blood levels of CK, an indicator of muscle damage, at rest pre-GTX and 60 min of recovery post-GTX led to a decrease in DNA damage in the lymph. Specifically, differences were found in CK levels at rest pre-GTX and 60 min of recovery post-GTX between the two sexes. At rest, CK levels were more effectively reduced in the female group than in the male group, and at 60 min of recovery post-GTX, the opposite was observed. Further studies are needed to confirm these findings. Urine 8-OHdG levels were significantly reduced at 60 min of recovery post-GTX after training in both male and female groups. Specifically, 8-OHdG levels were significantly lower in the female group than in the male group.
As such, reduced muscle and DNA damage after HIIE appears to be achieved by improved physical strength and adaptation after 8 weeks of training, which subsequently increased defense against stress and upregulated recovery processes against DNA damage. However, these results do not suggest the exact mechanism for the differences in DNA damage by sex. Future studies must consider various factors that can affect DNA damage, including metabolic mechanisms, hormonal effects, exercise intensity, and exercise duration according to the fitness level of the participants.
ACKNOWLEDGMENTS
The authors received no financial support for this article.
Notes
CONFLICT OF INTEREST
No potential conflict of interest to this article was reported.