Functional assessment of respiratory muscles and lung capacity of CrossFit athletes
Article information
Abstract
CrossFit is a high-intensity training related to physical fitness and respiratory capacity that can promote changes in lung function. This cross-sectional study was aimed at evaluating respiratory muscle strength, electromyographic (EMG) activity, and lung capacity in CrossFit athletes. Thirty subjects aged between 25 and 35 years were divided into groups: CrossFit athletes (n=15) and sedentary individuals without comorbidities (n=15). Respiratory muscle strength was evaluated using maximal inspiratory and expiratory pressures, lung capacity, and EMG of the sternocleidomastoid, serratus anterior, external intercostal, and diaphragm muscles at respiratory rest, maximal inspiration and expiration, and respiratory cycle. Data were tabulated and subjected to statistical analyses (t-test and Spearman test, P<0.05). Respiratory muscle strength on EMG of the sternocleidomastoid, serratus, external intercostal, and diaphragm muscles at the respiratory cycle and maximal forced inspiration and expiration were higher in the CrossFit athletes group than in the sedentary group without comorbidities. CrossFit athlete group showed significantly strong positive correlation between maximal inspiratory and expiratory muscle strengths (Spearman rho= 0.903, P=0.000), with increasing muscle strength during inspiration favoring an increase in strength during expiration. The forced vital capacity (FVC) and forced expiratory volume in 1 sec (FEV1) also showed a significantly high positive correlation (Spearman rho=0.912, P=0.000) in the CrossFit athletes group, showing that higher FVC favors higher FEV1. The results of this study suggest that improved fitness is based on increased respiratory muscle strength on EMG in CrossFit athletes.
INTRODUCTION
CrossFit is considered to be a multimodal physical training that covers functional movement patterns in a single session, performed at high intensity, involving strength and conditioning (Forte et al., 2022; Moran et al., 2017). Each session has variations, with an average duration of 1 hr, and comprises specific warm-up, skilled exercises, programmed strength and conditioning training for 10–30 min, cool-down, and mobility exercise (Butcher et al., 2015). The improved metabolic capacity provided by CrossFit increases the variables that determine lung function, since the duration, type, and intensity of exercise affect the respiratory system (Losnegard and Hallén, 2014). Recent advances in technological and scientific observations have provided athletes and coaches with several options to improve training (HajGhanbari et al., 2013). The search for alternative techniques to classical training methods, such as resistance and cardiovascular exercise, determine functional parameters aimed at improving performance or accelerating the recovery of athletes (Rose et al., 2017).
Scientific and clinical alternatives for evaluating performance includes respiratory muscle strength, which allows the prescription of training load and reflects the pressure developed by the inspiratory muscles, in addition to the passive elastic recoil of the chest wall and muscles involved in this dynamic (Çelik et al., 2022). A muscle strength assessment can be complemented or associated with measures of lung capacity and respiratory muscle activity, assessed using spirometry and electromyography (Graham et al., 2019). From these evaluation variables, determining the appropriate training intensity and the presence of respiratory muscle weakness is possible. Respiratory muscle strength reduces after participation in various sports, such as rowing, running, and cycling (Romer et al., 2002; Tong et al., 2014).
Therefore, the aim of the present study was to evaluate the performance of the respiratory system in CrossFit athletes, as a way of understanding the possible physiological changes resulting from the practice of the modality, since changes in the respiratory system have been reported. The hypothesis of this study is that respiratory muscle strength, lung capacity, and electromyographic activity of respiratory muscles can be performance indicators of CrossFit, with the possibility of correlations among the variables studied.
MATERIALS AND METHODS
Ethical approval
The cross-sectional study was reviewed and approved by the ethics committee of the University of São Paulo at Ribeirão Preto Dental School, São Paulo, Brazil (process # 3.551.119). All participants were informed about the protocol and potential risks and signed an informed consent form. Authors declare that the study reported were performed in accordance with the ethical standards of the Helsinki Declaration.
Sample selection
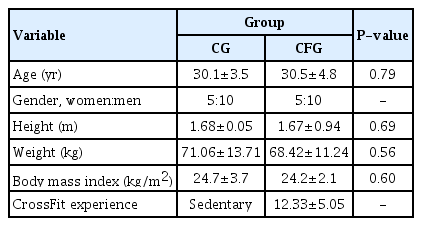
The sample size was calculated according to a previous study (Sprey et al., 2016), considering the last update of the CrossFit population. The t-test was performed at α=0.05, with a test power of 85% and an effect size of 0.90, using the variable body weight as the mean and standard deviation of the group that suffered injury or not. Finally, the minimum sample size was 15 participants in each group. The sample size was calculated using G*Power 3.1.9.6 software (Franz Faul, Kiel University, Kiel, Germany). From a total of 60 participants evaluated, following the inclusion and exclusion criteria, 30 were selected and divided into two groups: CrossFit athletes (n=15; 5 women and 10 men) and sedentary individuals without comorbidities (n=15; 5 women and 10 men). The groups were matched by age, sex, and body mass index (Table 1).
Inclusion criteria were age between 25 and 35 years and no pulmonary involvement. Participants who presented with ulcerations, open wounds or cutaneous hypersensitivity, presence of cognitive deficits, other neurological or systemic (decompensated) pathologies, or use of analgesics or muscle relaxants that could interfere with neuromuscular physiology and smokers who presented with rib cage deformities were excluded. Severe scoliosis and signs of influenza during the week of assessment were also exclusion criteria. The CrossFit routine showed functional movements with high sustained intensity and constant variation during exercise, maintaining the pattern every day. The trained instructor determined a sequence of exercises, and all participants followed the same protocols. Data were collected from athletes who attended gyms with the same standard of physical training.
Maximal respiratory strength
An analog manovacuometer with a range of ±300 cmH2O (Murenas, Juiz de Fora, Minas Gerais, Brazil) was used to measure maximal respiratory pressures as quantitative changes in respiratory muscle strength (Black and Hyatt, 1969; Silva Andrade et al., 2022). Expiratory pressure was calculated from the total lung capacity, represented by the maximum expiratory pressure (MEP), and the maximum inspiratory pressure (MIP) was measured at the residual volume level. The participant was positioned in a comfortable chair in Fowler’s position, with the upper limbs beside the body and the lower limbs flexed at a 90° angle. The mouthpiece of the device was adapted to the participant’s oral cavity with the nose occluded using a nose clip. Verbal commands were delivered by a single trained evaluator, instructing the participant to fully exhale, try to empty the lungs as much as possible, and then inhale deeply and quickly through the mouth. MIP was measured from the residual volume. The mouthpiece of the device was again attached to the participant’s mouth with the nose occluded using a nose clip, and the participant was instructed to inhale completely, trying to fill the lungs as much as possible. The command was then delivered to exhale deeply and quickly through the mouth. MEP was measured using total lung capacity. The procedures were performed thrice, with a measurement interval from one pressure to another of 1 min, considering the highest pressure valid.
Vital capacity and forced expiratory volume
Vital capacity and forced expiratory volume were analyzed using a spirometer, in accordance with the technical procedures, acceptability, and reproducibility criteria, in an air-conditioned room environment between 22ºC and 24ºC, according to the guidelines laid down by the American Thoracic Society/European Respiratory Society (Miller et al., 2005). The participants were instructed to avoid bulky meals and not smoke or drink alcoholic beverages, coffee, or tea on the day of the examination. During the test, the participants remained seated with the elbows, hips, and knees at 90º, applied the nose clip, and received instructions on the respective maneuvers before performing the procedures. The lips were adjusted to the mouthpiece to prevent air leakage. Deep inspiration was performed, followed by rapid and forced expiration for as long as possible. At the end of the maneuver, deep inspiration was performed again. During the maneuvers, constant and repetitive stimuli of the instructor responsible for the examination were important. A minimum of three and a maximum of five forced expiratory curves were obtained to measure the forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1) in L, and the relationship between these two variables (FEV1/FVC) as a percentage (%).
Electromyographic activity of the respiratory muscles
Electromyographic activities of the sternocleidomastoid (right and left), external intercostal, diaphragm, and anterior serratus muscles were analyzed using the MyoSystem BR1 P84 portable electromyograph (DataHominis, Uberlândia, Minas Gerais, Brazil). During the electromyographic examination, the environment was kept silent, with the participant seated without shoes on rubber mats asked to remain as calm as possible and breathe slowly. The participant’s head was positioned upright, with the face facing forward and looking towards the horizon. Instructions and necessary explanations were provided, asking the participant to remain calm (Moreto Santos et al., 2020). The surface electrodes were positioned according to the recommendations of the Surface EMG for Non-Invasive Assessment of Muscles project (Hermens et al., 2000). Before placing the electrodes, the skin was cleaned with alcohol to reduce impedance (Di Palma et al., 2017). To determine the placement of the electrodes in collecting the electromyographic signal from the respiratory muscles, a maximum voluntary pressure maneuver was performed during inspiration (MIP) and expiration (MEP) (Xu et al., 2017). The arrangement of the electrodes for collecting the diaphragm muscle was based on the positioning of the midclavicular line of the 6th intercostal space (Chien et al., 2010). Electromyographic evaluation was performed with the participant in a seated position, with the upper limbs beside the body, and the lower limbs flexed at a 90° angle. Respiratory function was analyzed at respiratory rest, respiratory cycle (deep inspiration and expiration), and maximum inspiration and expiration, with a 1-min interval between collections. The respiratory muscles of the left side of the body could produce crosstalk owing to cardiac interference in the acquisition of the electromyographic signal; therefore, to avoid impedance when capturing the signal to the external intercostal, serratus anterior, and diaphragm muscles, collection was performed only from the right side of the body (Abbaspour and Fallah, 2014; Hawkes et al., 2007). The protocol for normalizing the electromyographic recordings of the respiratory muscles was followed using the maximal inspiration maneuver sustained for 4 sec.
Statistical analysis
Data were analyzed using the IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA). The collected data were evaluated using descriptive analyses (mean±standard deviation). Data distribution was verified using the Kolmogorov–Smirnov test (P=0.200). Statistical significance was determined using t-tests (P<0.05). The correlation between respiratory variables and electromyographic activity was analyzed using the Spearman test (P<0.05) after the Levene test for homogeneity of variance and linear relationship analysis.
RESULTS
Table 2 shows the mean respiratory muscle strengths (MIP and MEP) and lung capacities (FVC, FEV1, and FEV1/FVC) of the groups. The CrossFit athlete group presented higher averages, with significant differences in MIP (P=0.000) and MEP (P=0.000). FVC, FEV1, and FEV1/FVC showed no differences between the groups.

Differences in mean values (±standard error) of respiratory muscle strength and lung capacity between groups
Table 3 shows the electromyographic means of the sternocleidomastoid (right and left), external intercostal, diaphragm, and serratus anterior muscles at respiratory rest, respiratory cycle, and maximal inspiration and expiration. The results at respiratory rest did not differ significantly between the groups for all muscles. In the maximum inspiration condition, the CrossFit athlete group showed significant differences with higher electromyographic means in the external intercostal (P=0.03), serratus anterior (P=0.01), diaphragm (P=0.000), right sternocleidomastoid (P=0.002), and left sternocleidomastoid (P=0.000) muscles. In the maximum expiration condition, the CrossFit athlete group showed significant differences with higher electromyographic means in the external intercostal (P=0.02), serratus anterior (P=0.01), and diaphragm (P=0.001) muscles. In the respiratory cycle, the CrossFit group showed higher electromyographic means, with significant differences in the serratus anterior muscle (P=0.02).

Differences in mean±standard error of normalized electromyographic activity of respiratory muscles between groups
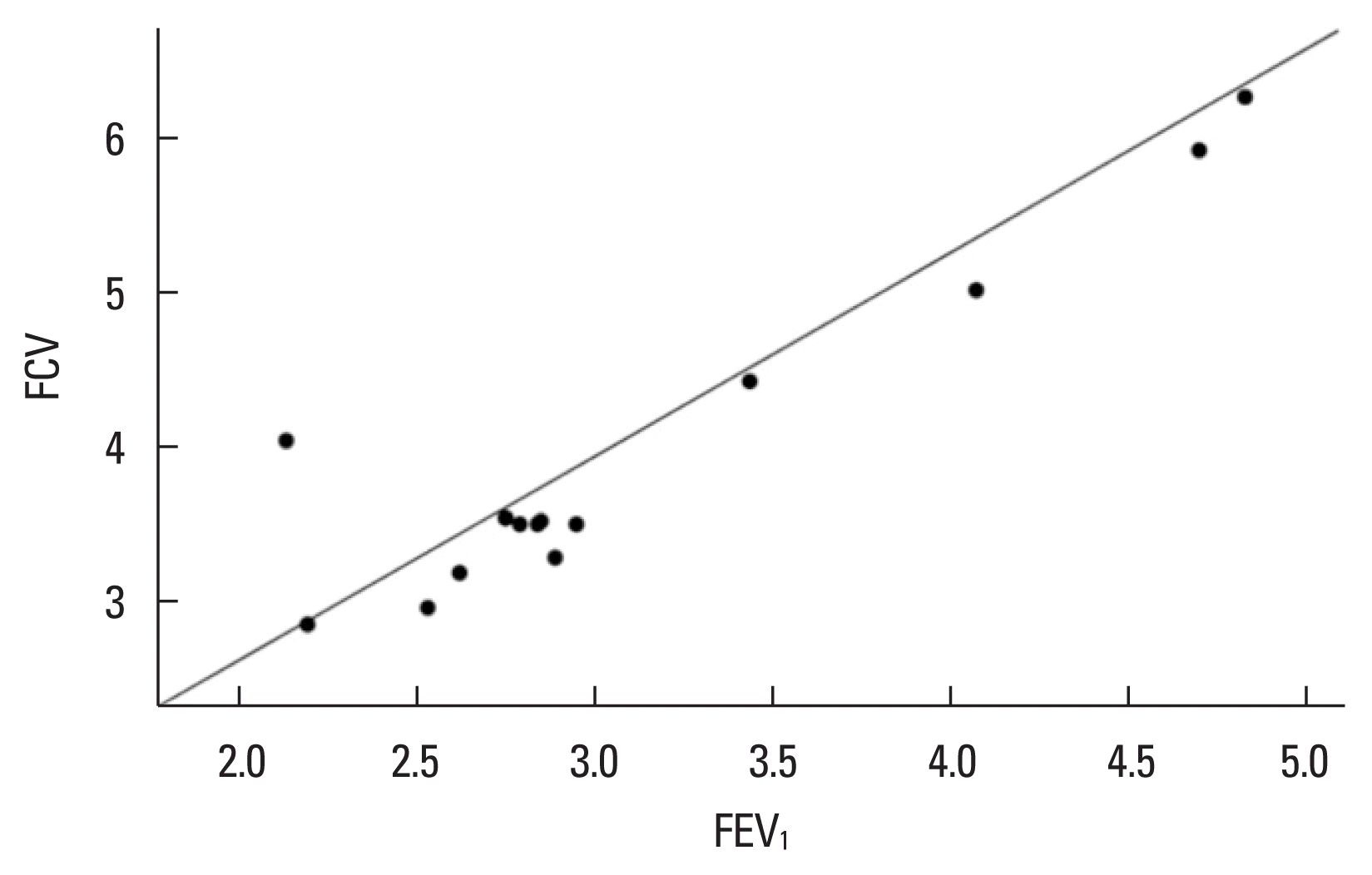
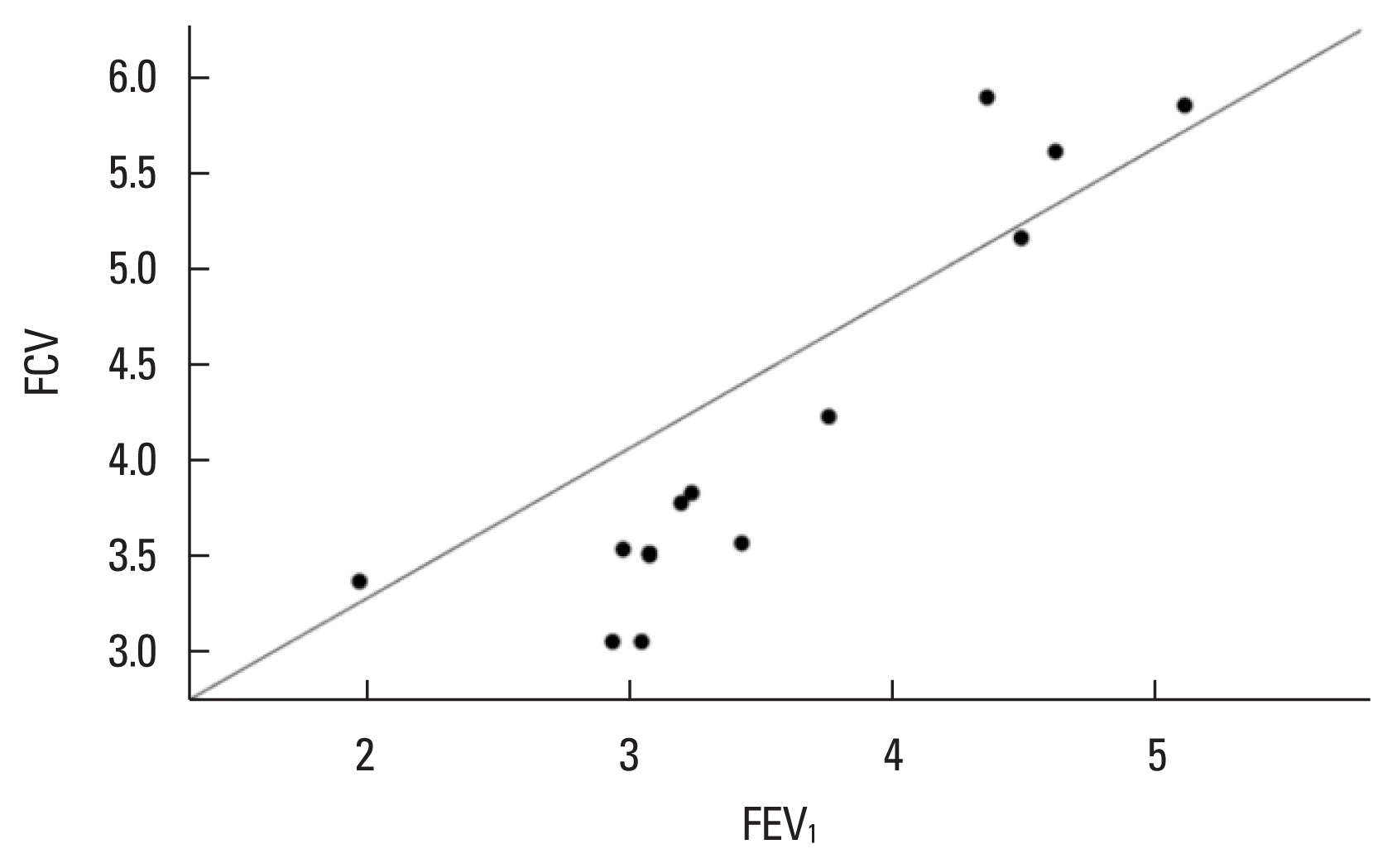
The CrossFit group demonstrated a significantly high positive correlation between MIP and MEP (Spearman rho=0.903, P= 0.000, Fig. 1). FVC and FEV1 showed a significantly high positive correlation in the CrossFit group (Spearman rho=0.912, P=0.000, Fig. 2) and a moderately significant positive correlation in the sedentary group without comorbidities (Spearman rho=0.637, P=0.01, Fig. 3).
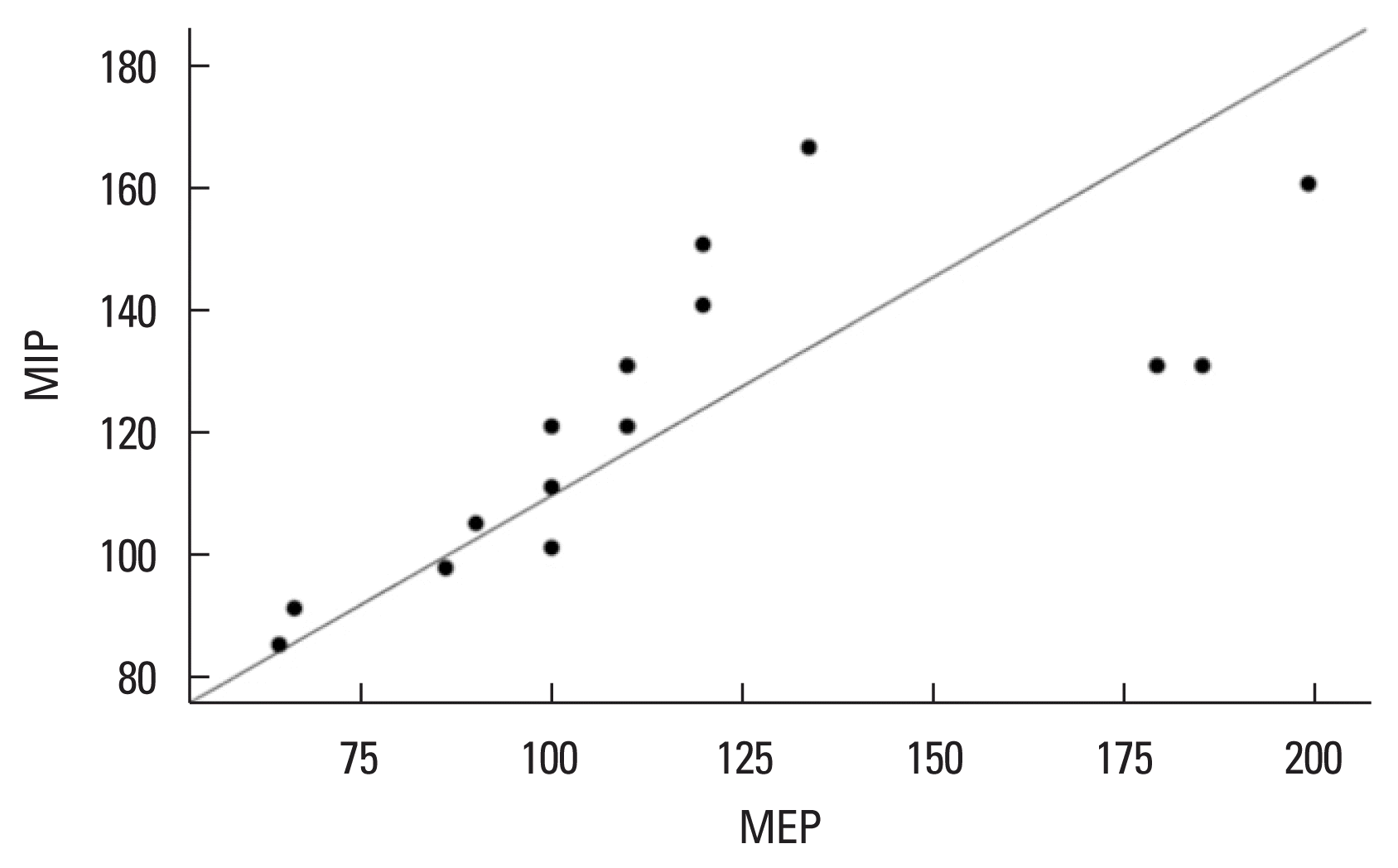
Correlation between MIP and MEP in the CrossFit athlete group. MIP and MEP showed a high positive correlation (rho=0.903; P=0.000). MIP, maximum inspiratory pressure; MEP, maximum expiratory pressure.
Correlation between FVC and FEV1.in the CrossFit athlete group. FVC and FEV1 showed a high positive correlation (rho=0.912, P=0.000). FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec.
DISCUSSION
The hypothesis that the CrossFit athletes would show improvement in physical fitness was supported by the results of this study. However, respiratory muscle strength and activity showed no correlation. Respiratory muscles are of vital importance in the performance of athletes and significantly influence their exercise tolerance. This study showed that the CrossFit athlete group had higher MIP and MEP than the sedentary group without comorbidities. These findings are reflected in other groups of elite athletes, such as practitioners of judo, rowing, gymnastics (Akınoğlu et al., 2019). A hypothesis that would explain the high results in the CrossFit athlete group would be enhancement of the muscle metaboreflex, which increases oxygenation and blood supply in the body’s peripheral musculature (Archiza et al., 2018).
Physical training using CrossFit requires a certain range of effort patterns, which demonstrates a greater connection with glycolytic metabolism. These, in turn, represent activities that depend on technical gestures to achieve better performance and use of explosive movements (anaerobic lactic and glycolytic systems), as repeated sprints present effort levels close to or greater than the second ventilatory threshold (Guy et al., 2014). CrossFit exercise models and effort levels can mimic the effects of respiratory muscle training; therefore, athletes in this sport category can improve respiratory muscle strength. Study suggests that strengthening the respiratory muscles of elite swimmers, who performed sports training associated with respiratory muscle training with specific equipment, and a group that performed sports training without the use of equipment, showed similar results (Mickleborough et al., 2008).
These findings reinforce the results of this study, in which the effects of CrossFit on respiratory muscle strength were observed without using any linear or nonlinear loading device. In contrast, another study showed that showed that using devices for respiratory muscle training for 4 weeks by basketball players improves the strength and resistance of the respiratory muscles, reducing respiratory muscle fatigue and prolonging the metabolic restriction reflex that provides the musculoskeletal system with greater energy availability (Tranchita et al., 2014). Therefore, achieving this effect without using these devices during CrossFit is a low-cost and effective alternative. These findings are important because this study showed positive correlation data between MIP and MEP in the CrossFit athlete group; in other words, the increase in inspiratory muscle strength can improved forced expiration involving the abdominal muscles.
In relation to these aspects, the sedentary group without comorbidities also showed a positive correlation between MIP and MEP. Therefore, this group can benefit from respiratory muscle training and prepare to engage in activities or exercises that have physical fitness requirements as characteristics. To understand the influence of activity following exhaustive protocols on the respiratory muscles, electromyographic examinations are essential. Study has shown, through electromyographic data of healthy participants, that 3 weeks were sufficient to attenuate muscle fatigue using incremental exercise of respiratory training, suggesting neural adaptation (Segizbaeva et al., 2015). Respiratory muscle training improves physical performance; however, they did not address any type of respiratory muscle training. This may lead to a reflection on where CrossFit practitioners could benefit even more from the performance effects associated with respiratory muscle training (Illi et al., 2012). The use of respiratory muscle training associated with cycling training increases the electromyographic activity in the diaphragm and sternocleidomastoid muscles, suggesting that this association provides a functional summation effect to the training (Hellyer et al., 2015). These data corroborate the findings of this study in relation to the CrossFit athlete group. This situation could be justified by training that aims to promote greater recruitment of muscle fibers, greater respiratory muscle strength, and increased lung capacity (Claudino et al., 2018; Mahler and Loke, 1985).
Elite athletes have higher MIP and MEP compared to participants who do not practice sports and are healthy, although the results vary across sports (Ohya et al., 2016). However, the benefits of CrossFit training present related responses of respiratory muscle strength assessed by maximal inspiratory and expiratory pressures (Rose et al., 2017). When evaluating the functional respiratory capacity of an athlete, the diaphragm musculature is more developed, as it contributes approximately 70% to 90% of the total production of inspiratory pressure throughout exercise (Hellyer et al., 2015). These data are consistent with the findings of this study, which demonstrated greater electromyographic activities for the respiratory muscles in the CrossFit athlete group during forced inspiration and expiration, compared to the sedentary group without comorbidities, since the level of vascularization and redistribution of blood flow of practitioners must be well structured in adapting to training periods (Claassen et al., 2021). Despite the good performance of respiratory muscles, this study did not demonstrate improvements in pulmonary function variables when comparing CrossFit athlete and sedentary groups without comorbidities. It is known that after the complete biological development of a participant, the lung size does not change because of the influence of sports practices; therefore, the spirometric values do not change (Martinez-Navarro et al., 2021).
As our study was carried out in athletes with a mean age of 30 years, that is, participants with optimized lung function, changes in lung function are less likely after a period of lung development and maturation. Analogously, this would be the same as expecting a training program to lead to an increase in height of participants who have reached their maximum height. Although exercise cannot change lung size, it can, in some cases, improve lung function, particularly in sedentary participants and those athletes with existing conditions that specifically impair lung function (Roman et al., 2016). For this to occur, the exercise modality must first acutely affect these parameters and their responsible mechanisms to ensure progressive overload and adaptation (Jones et al., 2017). Although this study did not find acutely altered pulmonary function variables (FVC and FEV1), the CrossFit athlete group showed differences when compared to the sedentary group without comorbidities.
Another point of discussion is that athletes have excellent pulmonary function resulting from the high ventilatory effort during periods of training or competition and for long years of practice (Durmic et al., 2015). Thus, the variables evaluated possibly did not benefit from the comparison between groups. In within-group comparisons of this study, the CrossFit athlete group showed a high positive correlation, and the sedentary group without comorbidities showed a moderate positive correlation when FVC and FEV1 were analyzed; that is, despite these variables not being affected by the strength of the respiratory muscles, if the FVC increased, FEV1 was likely to increase. This observation is important because FEV1 is generally considered an effort-dependent pulmonary parameter, and higher values depend on the abdominal muscles, which are mainly active during expiration (Molgat-Seon et al., 2022). Although not similar, studies on the effect of exercise on lung function have been performed who observed improvement in FVC and FEV1 in participants with overweight and obese after 24 weeks of exposure to aerobic exercise (Azad et al., 2011). This study has some limitations. A more assertive analysis of lung function differences was not performed using maximum voluntary ventilation, which would help to establish the fatigue threshold between CrossFit athletes and sedentary participants without comorbidities, allowing more information in terms of training and protection mechanisms that would probably prevent exhaustion of respiratory function.
CrossFit was created for athletic performance in well-trained participants; therefore, the results of this study suggested an improvement in physical fitness, since exercise using CrossFit increased the strength of the respiratory muscles and the electromyographic activity of the muscles involved in breathing without major effects on lung capacity. CrossFit athletes can benefit from the positive correlations between inspiratory and expiratory muscle strengths since the increase in inspiratory muscle strength provides better rates of expiration.
ACKNOWLEDGMENTS
This study was supported by a grant that was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the National Institute of Science and Technology in Translational Medicine.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.