Virtual reality-based gait rehabilitation intervention for stroke individuals: a scoping review
Article information
Abstract
Virtual reality (VR)-based rehabilitation is rapidly gaining interest and has been shown to be an intervention to facilitate motor learning in balance and gait rehabilitation. A review of the current literature is needed to provide an overview of the current state of knowledge of VR-based gait physiotherapy for stroke patients. A systematic literature search was performed in PubMed and Scopus. Search terms included: “virtual reality,” “stroke,” “gait,” and “physical therapy.” Articles published in a peer-reviewed journal between 2017 and 2021 were considered. The intervention was mainly related to the use of VR as a therapeutic modality, and the outcome was gait performance. The initial search identified 329 articles. After an eligibility review, 13 articles that met the inclusion criteria were included in the study. Most of participants were in a chronic stage and were between 14 and 85 years old. The VR-based gait training ranged from nonimmersive to immersive, was mostly performed on a treadmill, and was usually combined with conventional physiotherapy. The duration of the program varied from 10 to 60 min, and there were about 9 to 30 sessions. VR-based gait rehabilitation has a positive effect on gait ability. The existing literature suggests that VR-based rehabilitation combined with conventional physiotherapy could improve gait ability of people with stroke, especially in the chronic stage. However, the duration of VR-based programs should be customized to suit individuals to avoid stimulation sickness. Further research is needed to investigate the long-term effects of this approach.
INTRODUCTION
Walking impairment affects >80% of stroke survivors (Duncan et al., 2005). Due to the disruption of neural networks in the motor cortex, their communication with the brainstem and its descending pathways, and the intraspinal locomotor network, abnormal gait patterns are a common disability after stroke. This damage leads to weakening of the muscles, changes in tone, and atypical synergistic movement patterns that are commonly seen in stroke patients (Li et al., 2018). Difficulty in walking may increase due to secondary impairments of the cardiovascular and musculoskeletal systems caused by physical inactivity. After a stroke, the gait pattern of affected individuals often consists of both new compensatory movement patterns specific to their injury and movement abnormalities (Balaban and Tok, 2014). Approximately 25% of stroke survivors have residual gait deficits in walking that require full physical support by the time they are discharged from the hospital, despite attempts at rehabilitation (Hendricks et al., 2002). As a result, gait impairment makes it difficult to perform activities of daily living and improve functional mobility (Li et al., 2018). To date, there are various gait rehabilitation interventions, including treadmill training with or without body weight support, robotic-assisted therapy, circuit training, self-rehabilitation programs, and virtual reality (VR) (Selves et al., 2020).
VR is a relatively new tool in the field of physical rehabilitation (Dockx et al., 2016; Tieri et al., 2018) and can be defined as “an artificial, computer-generated simulation or creation of a real-life environment or situation allowing the user to navigate through and interact with” (Baus and Bouchard, 2014). VR provides a safe, supervised environment for engaging, customizable rehabilitation activities that support motor skill acquisition (Aida et al., 2018). The potential of VR-based interventions to provide meaningful and realistic experiences, thereby accommodating rehabilitative principles, may explain the remarkable improvement in rehabilitation outcomes of patients who have participated in VR-based interventions (Levin, 2011). When tasks are meaningful, specific, and repetitive and the level of difficulty increases over time, learning improves (Kleim and Jones, 2008; Levin, 2011). While maintaining stimulus control and consistency, the number of stimuli and the difficulty of tasks can be changed at VR to meet patients’ needs and abilities (Maier et al., 2019a; Rizzo and Koenig, 2017). The systems of VR can send real-time strategic and goal-directed feedback, which is important for motor learning and accelerates self-correction (Ferreira et al., 2020; Pedreira et al., 2017). Therefore, the aim of this review was to assess the current state of information on the effects of VR on the gait performance of people after stroke from the current literature.
MATERIALS AND METHODS
This scoping review was conducted with the aim of identifying existing research findings and trends in the use of VR-based interventions to improve gait performance in stroke patients. The study protocol followed an article entitled “Guidance for conducting systematic scoping reviews” (Peters et al., 2015).
Search strategy
A systematic literature search was performed in PubMed and Scopus that included the following search terms: “virtual reality,” “stroke,” “gait,” and “physical therapy.” Articles published in English in a peer-reviewed journal between 2017–2021 were considered. The intervention was mainly on using VR as a therapeutic modality and the outcome was gait performance. Studies that were not published in English, were available only as abstracts, did not include individuals after stroke, or included a mixed etiology sample with no separate description of outcomes related to the stroke sample were excluded.
Study selection
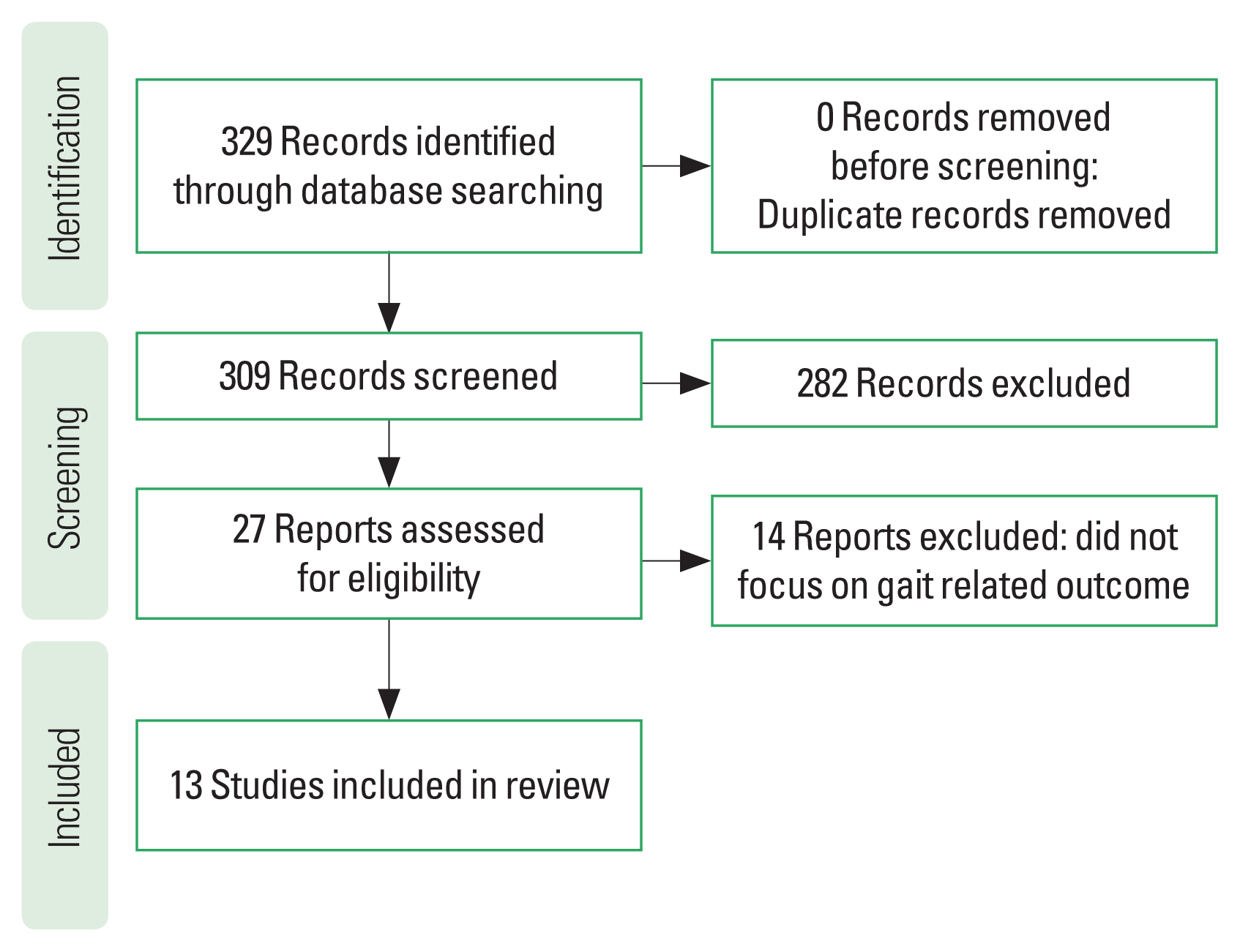
After database searches were completed, citations and abstracts were compiled and entered into Covidence, where duplicate citations were removed. In the first phase, two reviewers independently reviewed the titles and abstracts. Conflicts were discussed until agreement was reached between the reviewers. The two reviewers obtained full-text articles and screened them independently. Articles that met the inclusion criteria were included in the review. The details of the study selection process are shown in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) flow diagram (Fig. 1).
RESULTS
After the initial search, 329 records were identified. After removing 20 duplicates, 309 discrete titles and abstracts were screened for eligibility and 282 were excluded. The remaining 27 articles were selected for full-text reading, and 13 met our inclusion criteria.
Study context
Regarding the geographical context, the articles included in this review were mainly conducted in Europe, including two studies from Italy (Kiper et al., 2020; Luque-Moreno et al., 2021), two studies from Germany (Bergmann et al., 2018; Winter et al., 2021), one study from the Netherlands (de Rooij et al., 2021), and one study from Turkey (Kayabinar et al., 2021). Moreover, one study was conducted in a multi-setting in Spain and Switzerland (Held et al., 2018). The other geographic area included three studies from the Republic of Korea (Lee, 2019; Park and Chung, 2018; Park et al., 2021), one study from Israel (Fishbein et al., 2019), one study from Brazil (da Silva Júnior et al., 2021), and one study from Canada (Richards et al., 2018).
Participant characteristics
There were some differences in the characteristics of the participants in the included studies. Three studies recruited participants in the subacute phase (less than 6 months after stroke onset), six in the chronic phase, three recruited both subacute and chronic stroke participants, and one did not report chronicity. Most studies included participants aged 18 to 85 years. All included studies excluded participants with cognitive and/or visual impairment. Therefore, the typical sample in most studies consisted of middle-aged and older chronic stroke survivors without cognitive or visual deficits.
VR systems
This study included both custom-made and commercial VR systems used as interventions. Custom-made systems were typically laboratory-specific and often coupled other devices with the VR interface, such as robotic devices, treadmills, stationary bicycles, etc. For the commercial system, one study used a VR rehabilitation system (VRRS), a commercial medical device for rehabilitation. The other VR systems were the Sony PlayStation and Wii Fit. These devices are intended for the general public but are increasingly used in rehabilitation.
VR-based physiotherapy intervention
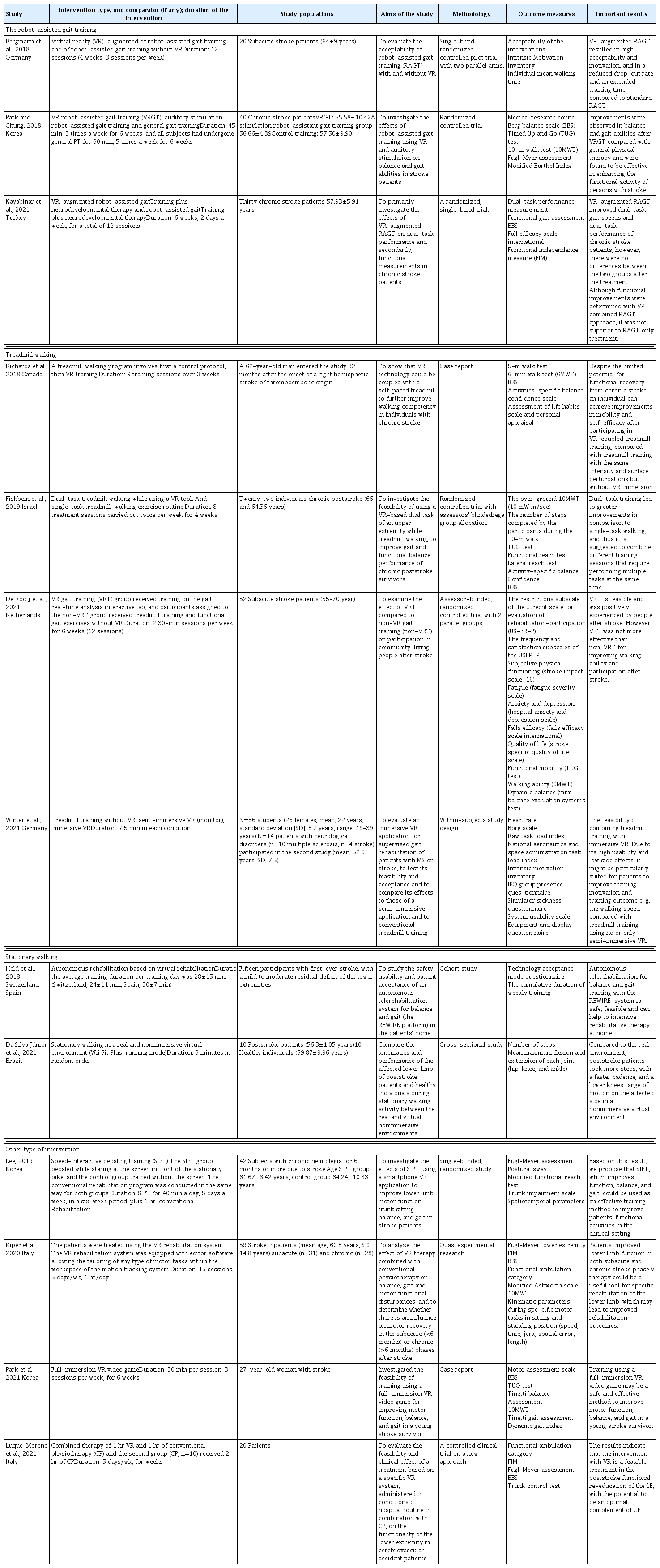
Among the 13 articles included in this review, the VR-based physiotherapy interventions could be classified into four categories: robot-assisted gait training, treadmill walking, stationary walking, and other interventions (Table 1).
Robot-assisted gait training
Three studies combined VR with robot-assisted gait training (RAGT). For example, Bergmann et al. (2018) used VR, to present a scenario in which participants had to walk a course displayed on the screen and solve various tasks by controlling the speed of the avatar by adjusting their motor activity while wearing the Lokomat robotic gait orthosis (Hocoma AG, Volketswil, Switzerland). The intervention lasted 12 sessions (4 weeks, 3 sessions per week). In a study by Kayabinar et al. (2021), VR was combined with RAGT 2 days per week for 6 weeks, for a total of 12 sessions. RAGT was performed with RoboGait (Middle East Technical University, Teknokent, Bama Teknoloji, Ankara, Turkey) and the two-dimensional game of RoboGait VR was played on the screen. The patients were tasked to walk in a forest environment with many trees without bumping into the trees and try to collect the coins appearing on the screen, using the scenario of collecting points in the system. Patients determined their direction during the game by transferring their weight to their extremities on the device. In another study by Park and Chung (2018), the Lokomat Pro (Hocoma AG, Zurich, Switzerland) was used for gait training. The VR function used a VR program called augmented feedback, a software embedded in the walking robot. In this VR program, the degree of mutual force between the patient and robot is represented by the movement of the avatar on the screen through the response of the sensors.
Treadmill walking
Four articles used treadmills for gait training. de Rooij et al. (2021) investigated the effect of gait training in VR by training participants on the gait real-time analysis interactive lab (GRAIL; Motekforce Link, Amsterdam, The Netherlands), which contains various rehabilitative applications in VR environments with specific rehabilitation goals (tasks). The difficulty levels were adjusted according to the individual abilities of the participants. The intervention took place for 6 weeks (12 30-min sessions). In a study by Fishbein et al. (2019), each training session began with an 8-min warm-up that included mobilization and flexibility exercises and a 2-min walk around the gym. Participants began walking slowly on a treadmill for 3 min. In subsequent phases, participants walked at the same speed while exercising using the three VR games. The total time walked on each trial was 20 min. Next, in a study by Richards et al. (2018), VR-based locomotion training was conducted using a walking simulator coupled with virtual environments back-projected onto a large screen. The participant, wearing a seatbelt and stereo goggles, walked on a motorized treadmill mounted on a six-degree-of-freedom motion platform, using a sliding handrail (to simulate the use of a cane), and progressed through virtual environment scenarios. Three levels of difficulty were set for the street crossing, gait, and park walk scenarios, in which the participant traveled 40 m. The participant then walked on the treadmill. For each program, participants attended nine training sessions over a 3-week period. Finally, in the study by Winter et al. (2021), a VR scenario called “Homecoming” was implemented using the Unreal Engine. The VR scenario is presented from a first-person perspective and aims to increase training motivation through an engaging storyline and gamification elements. As you walk on the treadmill, the world continuously becomes more fertile and colorful until it is completely rebuilt. For completing certain distances, users are awarded virtual stars as achievements in the virtual environment, accompanied by positive comments from the virtual companion. In the immersive VR condition, the virtual shoes were displayed at the position of the real feet. All participants took part in three conditions: No VR, semi-immersive VR, and immersive VR.
Stationary walking
Two studies used stationary walking with VR to improve gait performance. According to a study by Da Silva Júnior et al. (2021), stationary walking was performed with a walker in front and a chair behind the patient to ensure safety. A Nintendo Wii device connected to a 52-inch screen TV was used to allow participants to interact with the virtual environment and create a nonimmersive virtual environment. The Wii Fit Plus game (running mode) was used in the virtual environment. In this game, the avatar runs through a park-like landscape and meets other runners, passing them along the way. In order for the avatar to run, the participants should perform a stationary walking with the control attached to their torso. The participants performed stationary walking in a real and nonimmersive virtual environment (Wii Fit Plus–Running mode) for 3 min in randomized order. In a study by Held et al. (2018), it was reported that the patient exercised in front of the TV screen, and the movement was tracked by the Kinect camera and used to animate the avatar. In some exergames, a force plate is used to track the pressure point projected on the virtual floor of the game to provide feedback to the patient. In an exergame, the patient sees himself. Autonomic rehabilitation based on virtual rehabilitation was conducted at participants’ homes for 12 weeks.
Other types of interventions
Four articles could not be classified into the above themes including video game, VRRS, and pedal training. In a case report by Park et al. (2021), a full-immersive VR video game using Sony PlayStation VR (Sony Interactive Entertainment Inc., Tokyo, Japan) was used. The first game was Fruit Ninja, where the background was stationary. The second game was Everybody’s Golf, a game in which the player uses a controller to play golf in a realistic VR environment with the feeling of being on a real golf course. Each game was played for 15 min, and there was an approximately 5-min break while the game was changed and the device was set up. Training with a full-immersive VR video game lasted 30 min per session, 3 sessions per week, for 6 weeks, for a total of 18 sessions. Two clinical trials used the VRRS (Khymeia Group Ltd., Noventa Padovana, Italy) as an intervention. In the study by Kiper et al. (2020), participants sat or stood in front of a large computer screen. The electromagnetic sensor was positioned at different locations on the participant’s leg and represented the end effector of the task depending on the exercise goal (e.g., it was placed at the foot, ankle, knee, or hip depending on the virtual task to be performed). The VRRS allows the use of two sensors. Thus, one sensor was used as an end effector, and the second sensor detected insufficient position compensation. The VR treatment lasted 15 sessions, 5 days/wk, 1 hr/day. In another study by Luque-Moreno et al. (2021), therapy by VRRS included the performance of different types of motor tasks in which the patient had real objects as references (stairs, high objects, signs on the floor, etc.). In this process, he interacted with a virtual scenario in which the movements of lower extremities were monitored using the motion capturing system to guide the kinematic trajectories of movement in different tasks. The intervention consisted of 15 sessions of conventional physiotherapy (1 hr per day, 5 days per week). Lee (2019) used a stationary bicycle for pedaling training. The bicycle training was performed manually so that the patient could set his own speed. The patient started at a comfortable speed that he normally used for exercise. Then, the pace was set by the patient. The training lasted 40 min in total, including 5 min of warm-up stretching, 5 min of slow pedaling, 25 min of main exercise, and 5 min of cool-down. During the main exercise, each video for the Speed-Interactive Pedaling Training group consisted of 10 min, and participants were provided with two 10-min videos.
Outcomes
Outcome measures were divided into four categories depending on the focus of the measurement: gait performance, components of gait, psychological components, and other measurements. Outcome measures for gait performance were measures that directly assessed the performance or quality of walking. In the included articles, the 10-m walk test was used to measure walking speed, the 6-min walk test was used to assess cardiovascular endurance during gait task, the Timed Up and Go test was used to assess mobility performance, the functional gait assessment was used to assess postural stability during the walking task, and gait speed was measured separately from the 10-m walk test in some studies. The second category includes the components of walking. This category includes measurements that assess body components related to gait ability. Measurements included lower extremity muscle strength and kinematic functions such as speed, spatial error, gait length, balance, and motor function assessments such as the Fugl–Meyer Assessment and the Modified Ashworth Scale. The third category includes psychological components. In this study, some items included psychological aspects as outcome measures, such as intrinsic motivation, anxiety and depression, mood, and adverse event safety measures. The other measurement categories were activity of daily living measures such as the Barthel Index, Functional Independence Measure, and heart rate.
DISCUSSION
The aim of this review was to summarize the current literature on VR-based interventions for gait training in stroke patients to identify potential avenues for future research. Thirteen articles met the inclusion criteria. Existing literature suggests that incorporating VR-based rehabilitation into conventional physiotherapy may be effective in improving gait ability in stroke patients, particularly in the chronic stage
Based on these findings, many commercial VR systems have been used. The use of commercial VR for neurorehabilitation is gaining interest. It was also seen as a cost-effective intervention for home-based programs and reduced therapist time (Aliprandi et al., 2022).
Consistent with the principles of neurorehabilitation based on motor learning and brain plasticity mechanisms, elements such as repetitive practice, spaced practice, dosage, task-specific, goal-oriented practice, variable practice, increasing difficulty, multisensory stimulation, and explicit/implicit feedback (Maier et al., 2019b) were used to some degree in VR-based interventions in all included studies.
With respect to walking tasks in VR-based intervention, such as stationary walking, walking on a treadmill, or robot-assisted walking, improved gait function outcomes were obtained. This could be due to the influence of task-specific or goal-directed training, in which patients practice context-specific motor tasks and receive some form of feedback (Teasell et al., 2008). Thus, task specificity seems to be a crucial, but not the only, factor in the use of VR-based interventions (Darekar et al., 2015). In addition, the intensity of the intervention could be considered as a dosage and timed exercise. In this study, the intensity of VR intervention ranged from 10–60 min per session for a total duration of 9–30 sessions. Additionally, some studies have used VR-based interventions in conjunction with conventional physical rehabilitation. Studies from neuroscience show that high-intensity rehabilitation protocols with prolonged training times can induce neuronal plasticity (Kwakkel et al., 2015) and reorganization of neural networks and improve motor functions (Veerbeek et al., 2014). In contrast to the previous scoping review (Darekar et al., 2015), this review also found that many studies included psychological outcomes such as immediate intrinsic motivation, anxiety, depression, and mood. These outcomes showed improvements in all participants. This could mean that the potential advantage of VR is that it provides highly repetitive training with great variability, which helps to maintain the patient’s motivation, limit their perception of effort, and improve their adaptability (Selves et al., 2020). Finally, regarding feedback, the majority of included studies used explicit feedback in the form of visual (e.g., success scores, stars) or auditory (e.g., cheers, feelings of success, and other sounds) feedback. Explicit feedback appears to activate explicit learning mechanisms while having minimal effects on implicit learning mechanisms (Taylor et al., 2014). Reinforcement of positive outcomes appears to promote a success-oriented learning system that limits decay after learning, possibly by mobilizing the dopaminergic system (Wickens et al., 2003). Implicit feedback was presented in the form of the quality of the movement, e.g., a graph or motion capturing system. Implicit sensory feedback enhances learning from sensorimotor prediction errors, which may aid in adaptation to unexpected perturbations (Shadmehr et al., 2010), possibly by contributing to implicit learning mechanisms (Taylor et al., 2014).
This is consistent with previous arguments that VR can provide meaningful and realistic experiences that facilitate rehabilitation success (Levac et al., 2019; Levin, 2011). The main advantages of VR are the accessibility of repeated practice, multisensory feedback, increasing task difficulty, and task specificity (Levac et al., 2019; Levin, 2011). Additionally, evidence from moderation analysis suggests that tailoring VR systems to patient needs can improve rehabilitation outcomes (Voinescu et al., 2021).
Overall, VR-based gait rehabilitation had a positive effect on gait components, gait performance, and psychological outcomes. However, one study reported that gait speed could not be measured due to the ground effect of the baseline performance. Serious adverse effects were not observed. In this study, it was found that the majority of stroke patients were in the chronic phase. Although spontaneous recovery usually reaches its limits in the chronic phase (Grefkes and Fink, 2020), this study found that positive improvement also occurs in participants in the chronic phase. This is consistent with previous systematic research that suggested that supplementing gait training with VR-based training could result in greater improvement in walking speed than non-virtual walking interventions (Rodrigues-Baroni et al., 2014).
However, this review was a scoping study and not a systematic review because no quality assessment of the included studies was performed. We found heterogeneity in the included studies in terms of participants, VR systems, VR-based intervention content, and outcome measures. Despite these limitations, initial findings were positive. The use of VR-based physiotherapy interventions for gait rehabilitation in stroke patients appear to be effective in improving gait components, gait performance, and psychological aspects.
In conclusion, existing literature suggests that VR-based rehabilitation combined with conventional physiotherapy can improve gait ability in people with stroke, especially in the chronic stage. The duration of the programs should be customized to avoid stimulation disease. Further research is needed to investigate the long-term effects of this approach.
ACKNOWLEDGMENTS
The authors received no financial support for this article.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.