Elastic band resistance combined with modified Thai yoga exercise to alleviate oxidative stress and airway inflammation in type 2 diabetes mellitus
Article information
Abstract
The objective of this study was to investigate the effects of the combination of elastic band resistance exercise (EBRE) with modified Thai yoga on the alleviation of blood glucose and oxidative stress in type 2 diabetes mellitus (T2DM). Forty-two patients with T2DM were enrolled and allocated to an exercise or control group (n=21/group). The exercise group participated in EBRE combination with modified Thai yoga for 40 min, 5 days/wk, for 12 consecutive weeks. Blood glucose, oxidative stress markers, antioxidants, pulmonary function, respiratory muscle strength, and airway inflammation were measured before and after the 12 weeks. The results showed that the exercise group had a significant reduction in fasting blood glucose and glycated hemoglobin. Moreover, T2DM patients in the exercise group showed a significant reduction in plasma malondialdehyde, while superoxide dismutase and catalase were significantly increased. The exercise group also observed a significant improvement in pulmonary function; forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, peak expiratory flow, and forced midexpiratory flow as well as respiratory muscle strength. Interestingly, the combination of EBRE with modified Thai yoga markedly improved airway inflammation through the reduction in fractional exhaled nitric oxide. In conclusion, these findings suggest that the combination of EBRE with modified Thai yoga improves blood glucose, oxidative stress, antioxidants, pulmonary function, respiratory muscle strength, and airway inflammation in T2DM patients. Hence, it could be considered as a possible exercise program for T2DM patients.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is one of the most common metabolic disorders which is an important cause of neuropathy, retinopathy, nephropathy, diabetic foot ulcers, coronary arterial disease, hypertension, and stroke (Balaji et al., 2019; Hou et al., 2020). The quality of life of patients with T2DM worsen when commodities coexist or complications appear (Trikkalinou et al., 2017). T2DM is a major global health problem that accounts for more than 90% of all diabetes in the world and tends to increase every year (Sun et al., 2022). The development of T2DM is primarily caused by a defective insulin secretion by pancreatic β-cells, or the incapacity of insulin-sensitive tissues to respond to insulin in term of is insulin resistance (Galicia-Garcia et al., 2020).
Oxidative stress is characterized by increasing reactive oxygen species, and/or reducing antioxidants leading to tissue damage. Hyperglycemia is the primary underlying pathophysiological mechanism connecting diabetes with oxidative stress (Fiorentino et al., 2013). Furthermore, hyperglycemia activates the protein kinase C, leading to the formation of reactive oxygen species and the elevated inflammation process (Xia et al., 1995). Nitric oxide (NO) is a powerful antioxidant. NO can be generated by three different isoforms of the enzyme NO synthase. The state of inflammation, numerous NO is produced by inducible NO synthase, and the amount of NO production was measured by using fractional exhaled NO (FeNO). In addition, NO also reacts with superoxide leading to increased reactive oxygen species and enhanced oxidative stress (Forstermann and Sessa, 2012).
Oxidative stress has been implicated in the development of insulin resistance, β-cell dysfunction, and vascular complications (Ceriello and Motz, 2004). Microvascular and macrovascular systems are compromised by DM which can lead to several issues that can damage the heart, nerves, kidneys, and eyes. What’s more, the lungs are a target organ for a condition known as diabetic lung (Pitocco et al., 2012) which is characterized by impaired lung functions such as reduced forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) (Lecube et al., 2017). Previous studies have suggested that T2DM may be a risk factor for decreased respiratory muscle strength, presumably because of microvascular problems, decreased muscle mass (Yuenyongchaiwat and Boonsinsukh, 2021), neuropathy of the phrenic nerve (Kolahian et al., 2019), impaired glycemic control (Al-Khlaiwi et al., 2021), and airway inflammation (Wellen and Hotamisligil, 2005).
The core of T2DM treatment is living a healthy lifestyle to maintain control of blood sugar levels, which includes increasing physical activity and eating a balanced diet. The importance of exercise training in the prevention and management of T2DM is becoming more widely acknowledged. Resistance training has been shown to significantly improve insulin sensitivity, mitochondrial function, lean body mass (Jansson et al., 2022), maximum oxygen uptake, FEV1, and FVC (Osho et al., 2012). Resistance training also decreased oxidative stress in T2DM (Vinetti et al., 2015). Additionally, exercise using elastic resistance bands have been reported to yield improvements in T2DM patients with regards to glycated hemoglobin (HbA1c), blood sugar, and lipid profiles (Park et al., 2016). Yoga is a feasible option for T2DM patients as it is less demanding on the cardiovascular system, and it is low impact, simple, and convenient (Larson-Meyer, 2016). Previous studies demonstrated that yoga exercise enhances muscle strength (Kanjirathingal et al., 2021), pulmonary function (Balaji et al., 2019), and antioxidant activity in T2DM (Hegde et al., 2011).
Although, resistance exercise and yoga have been well documented, beneficial outcomes among patients with T2DM, in addition to the effects of combined training, have not yet been reported. Therefore, the objective of this study was to investigate the effects of combined elastic band resistance exercise (EBRE) with modified Thai yoga on the alleviation of blood glucose and oxidative stress in T2DM. Furthermore, the combined effects of combination exercise on the improvement of pulmonary function, respiratory muscle strength, and airway inflammation were also determined in patients with T2DM.
MATERIALS AND METHODS
Patients and design
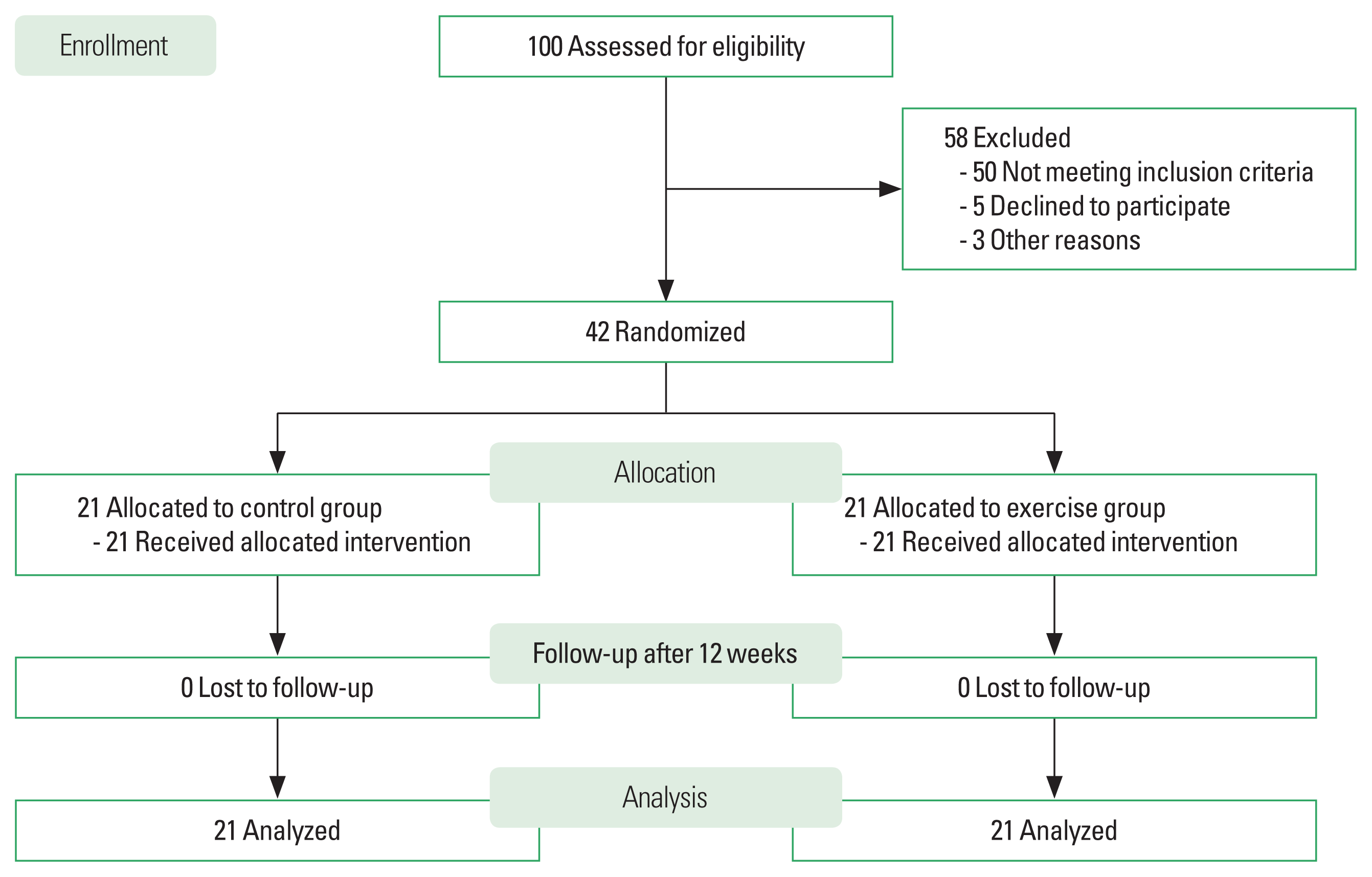
Sample size was calculated using a power of 0.90, power analysis with an effect size f of 0.78, and alpha of 0.05. The sample size was 20 per group. In addition, 5% dropout was calculated. Finally, the number of participants was 21 per group. This study was a randomized control trial. As shown in experimental design in Fig. 1, 100 older adults with T2DM were assessed for eligibility based on our inclusion and exclusion criteria. Forty-two elderly T2DM patients between the ages of 60 and 72 years participated in this study (20 males and 22 females). Participants were recruited in Phayao Province, Thailand from the Diabetes Clinic of a Subdistrict Health Promoting Hospital. A poster detailing the study was posted by staff at the center. Participants interested in participating in the study contacted staff or a research assistant via telephone. They had been diabetic for more than a year when they were given a T2DM diagnosis based on the American Diabetes Association Criteria. They had never done Thai yoga or EBRE prior. Insulin exposure, smoking, cardiovascular or neuromuscular disease history, physical activity limitations brought on by illness, a history of musculoskeletal injury during the previous 6 months, and involvement in any exercise program were all considered exclusion issues. Prior to the commencement of the study, written informed consent was received from each participant. Participants were randomly divided into two groups with an equal number: non-exercise group (control group) and the EBRE with Thai yoga (exercise group). The study was approved by the University of Phayao Human Ethics Committee (the approval number is 2/153/62).
Procedure
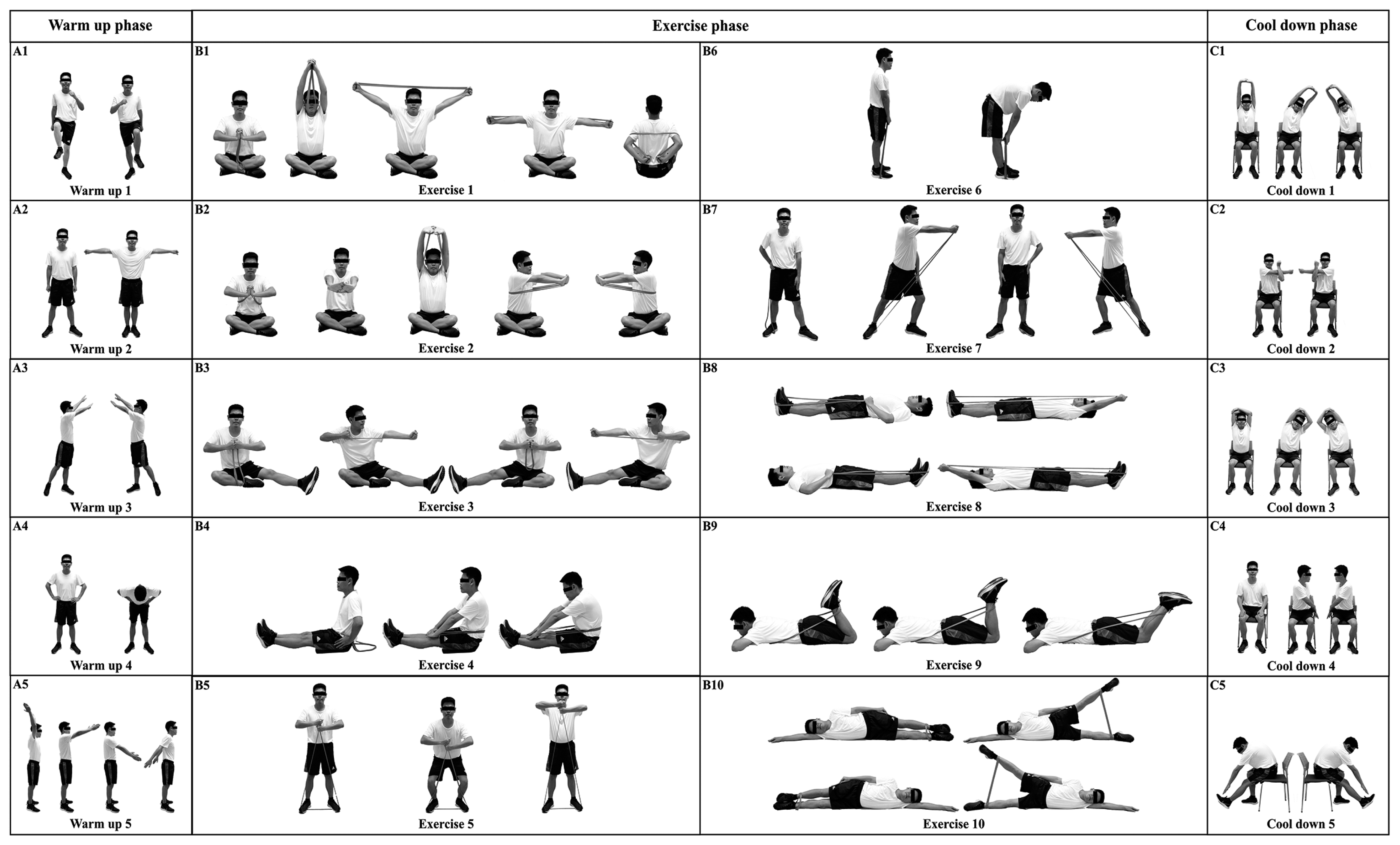
The control group (n=21) was instructed to carry on with their regular daily routine for 12 weeks. The exercise group (n=21) participated in EBRE with Thai yoga (40 min per day, 5 days per week) throughout the same 12-week period. The 40-min EBRE with Thai yoga has three phases: a 5-min warm-up phase (Fig. 2; A1–5), a 30-min exercise phase (Fig. 2; B1–10), and a 5-min cool-down phase (Fig. 2; C1–5). Participants completed each posture for one min during the warm-up phase, which included five different postures. Ten postures were included in the exercise phase, with individuals performing each posture for 3 min. Participants performed each of the five postures in the cool-down phase for 1 min. All the EBRE with modified Thai yoga postures were approved by an expert panel (Thai traditional medicine and physical therapist). An instructor introduced the EBRE with modified Thai yoga to participants in the exercise group 3 days a week for the initial 2 weeks. A weekly phone contact was made with every participant to inquire about their progress in the EBRE with modified Thai yoga. Before and after the 12-week period, anthropometric measurements, body composition, blood collection, pulmonary function, respiratory muscle strength, and FeNO were taken.
Outcome measurements
All outcome measurements were carried out by an experienced observer in our research group. The observer was also blinded for avoidance of bias.
Assessment of blood glucose and oxidative stress markers
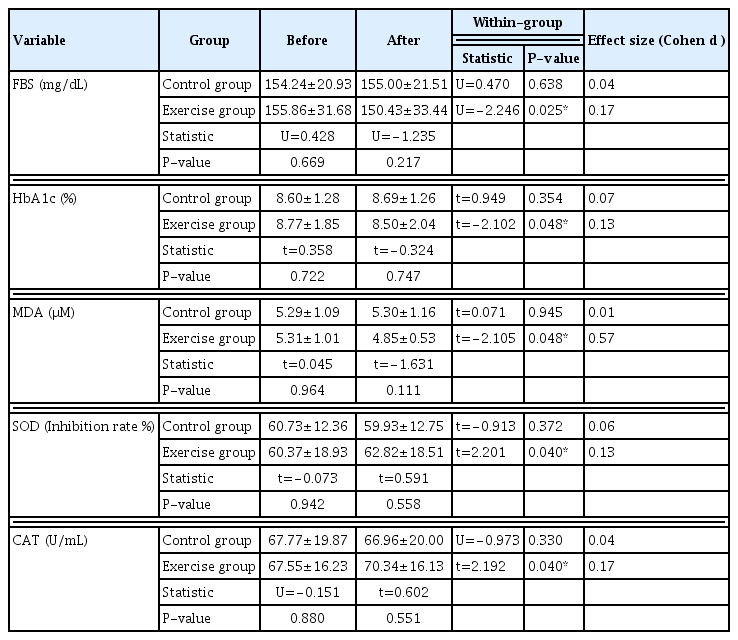
Fasting blood sugar (FBS), HbA1c, malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were measured in blood samples taken from the antecubital vein. FBS was measured by glucose oxidase technique using glucose reagent kits (DIRUI Industrial Co. Ltd., Changchun, Jilin, China) following the manufacturer’s instructions. Briefly, glucose in the blood sample was catalyzed by glucose oxidase of the reagent to form gluconic acid and hydrogen peroxide. Under the existence of peroxidase, hydrogen peroxide reacts with aniline and 4-aminoantipyrine to produce H2O and quinone imine pigment. The generated volume of quinone imine pigment is proportional to the glucose content in the blood sample. The final pigment volume was read at 505 nm. HbA1c was measured by HbA1c assay kits (Lifotronic, Shenzhen Lifotronic Technology Co. Ltd., Shenzhen, Guangdong, China), following the manufacturer’s protocol. Briefly, HbA1c monoclonal antibody (T line) and rabbit anti-mouse IgG were coated on the NC membrane, and the fluorescein labeled HbA1c monoclonal antibodies were coated on the conjugate pad. The blood sample was then added to the sample pad. The HbA1c reacts with the antibodies to form immune complexes. The concentration of immune complexes has a positive correlation to the concentration of HbA1c in the blood sample. HbA1c in the sample can be calculated through the standard curve. The level of plasma MDA was assessed utilizing the techniques as previously described (Somparn et al., 2007). MDA reacts with thiobarbituric acid in boiling water to form a colored complex called thiobarbituric acid-reactive substance, which can be detected by a spectrophotometric assay. Plasma samples (150 μL) were treated with 10% trichloroacetic acid, 5 mM ethylene diamine tetra-acetic acid, 8% sodium dodecyl sulphate, and 0.6 μg/mL of butylated hydroxytoluene. The mixture was incubated for 10 min at room temperature, 0.6% thiobarbituric acid was added, and the mixture was then boiled in a water bath for 30 min. After cooling to room temperature, the mixture was centrifuged at 10,000×g for 5 min. The absorbance of the supernatant was measured at 532 nm by a spectrophotometer. A standard curve was generated with appropriate concentrations of 1,1.3,3-tetraethoxypropane (0.3–10 μM). SOD activity in plasma was evaluated using a spectrophotometer and the corresponding detection kits (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions. Briefly, plasma was added to water soluble tetrazolium working solution and enzyme working solution then incubated at 37°C for 20 min. SOD content was read at 450 nm. CAT activity was determined as previously described by Goth (1991). Briefly, plasma was incubated with hydrogen peroxide in sodium potassium phosphate buffer at 37°C for 1 min. After that ammonium molybdate was added to stop the reaction. The yellow complex of molybdate and hydrogen peroxide was read at 405 nm.
Pulmonary function test
A pulmonary function test was performed by using spirometer (DATOSPIR touch, Sibelmed, Rosellón, Barcelona, Spain), following a spirometry standardization method according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines. The spirometer was calibrated using a 3-L syringe connected to the transducer as previously described following a spirometry normalization method. A calibration check of an expiration and inspiration volume report of less than 3% was considered acceptable. Subjects underwent pulmonary function testing while seated. A Spirometer was employed to measure pulmonary functions including FEV1, FVC, peak expiratory flow (PEF), and forced midexpiratory flow (FEF25%–75%). Measurements were taken from the greatest of three recordings made while the subject was seated and wearing a nose clip in accordance with the ATS/ERS recommendation. Values were represented as percentages of the predicted normal values (%predicted) in the Thai population.
Respiratory muscle strength
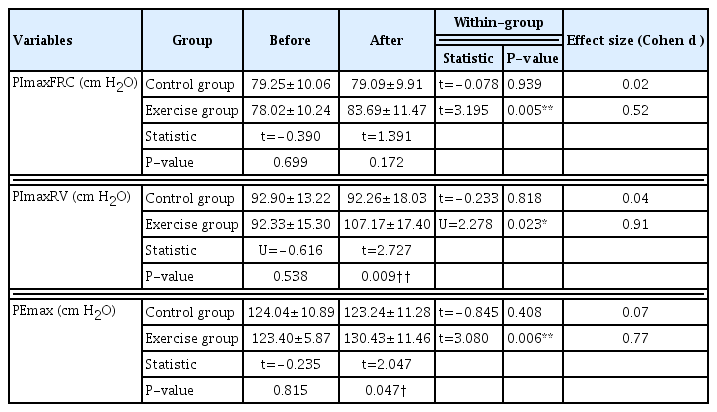
Maximal inspiratory pressure at functional residual capacity (PImaxFRC), maximal inspiratory pressure at residual volume (PImaxRV), and maximal expiratory pressure (PEmax) were measured to assess respiratory muscle strength using a respiratory pressure meter (MicroMedical MicroRPM 01, CareFusion, Basingstoke, UK) following the ATS/ERS guidelines. The instrument has been calibrated within 3% of a pressure manometer reading. The highest value among the three PImaxFRC, PImaxRV, and PEmax values were reported.
Fractional exhaled NO
FeNO was measured with the NO monitor (Quark NObreath, COSMED Srl, Pavona di Albano, Rome, Italy) with a single breath online method at a constant flow of 50 mL/sec for 12 sec of exhalation according to ATS/ERS guidelines, with a sensitivity of one part per billion (ppb). The software carried out the analyzer calibration automatically. The subjects inhaled to their total lung capacity, then exhaled through a mouthpiece into an exhalation circuit. Before the FeNO measurement, all volunteers were requested to abstain from eating, drinking, and performing intense exercise for 2 hr beforehand.
Statistical analysis
Data were presented as means±standard deviation. Stata 14.0 (StataCorp, College Station, Texas, TX, USA) was employed for all analyses. The Shapiro–Wilk test was utilized to test normal distribution of the data, paired t-tests were used to compare the data in the control and exercise groups before and after 12 weeks, while the unpaired t-test was used to compare between control and exercise groups. The Wilcoxon signed-rank and the Wilcoxon rank-sum tests were applied to evaluate data that were not normally distributed within and between groups, respectively. The effect size was calculated based on Cohen d. Statistical significance was defined as a P-value less than 0.05.
RESULTS
General characteristics of all participants
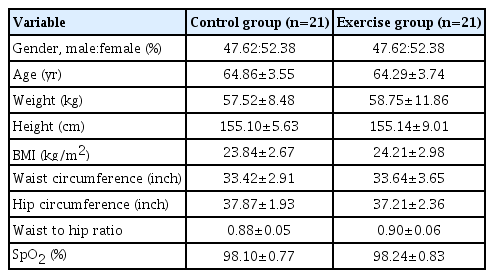
Forty-two T2DM patients aged 60–72 years, 20 males (47.7%) and 22 females (52.3%) took part in the study. All participants completed the study by participating in the 12-week assessment. There were no significant differences regarding baseline characteristic consists comprising of age, weight, height, body mass index, waist circumference, hip circumference, waist to hip ratio, and pulse oxygen saturation on comparison between the control and exercise groups (Table 1).
Primary and secondary outcomes
The primary outcomes of this study are blood glucose (FBS and HbA1c) and oxidative stress markers (plasma MDA, SOD, and CAT). Secondary outcomes include pulmonary function, respiratory muscle strength, and airway inflammation.
Effects of EBRE with modified Thai yoga on blood glucose and oxidative stress markers in T2DM patients
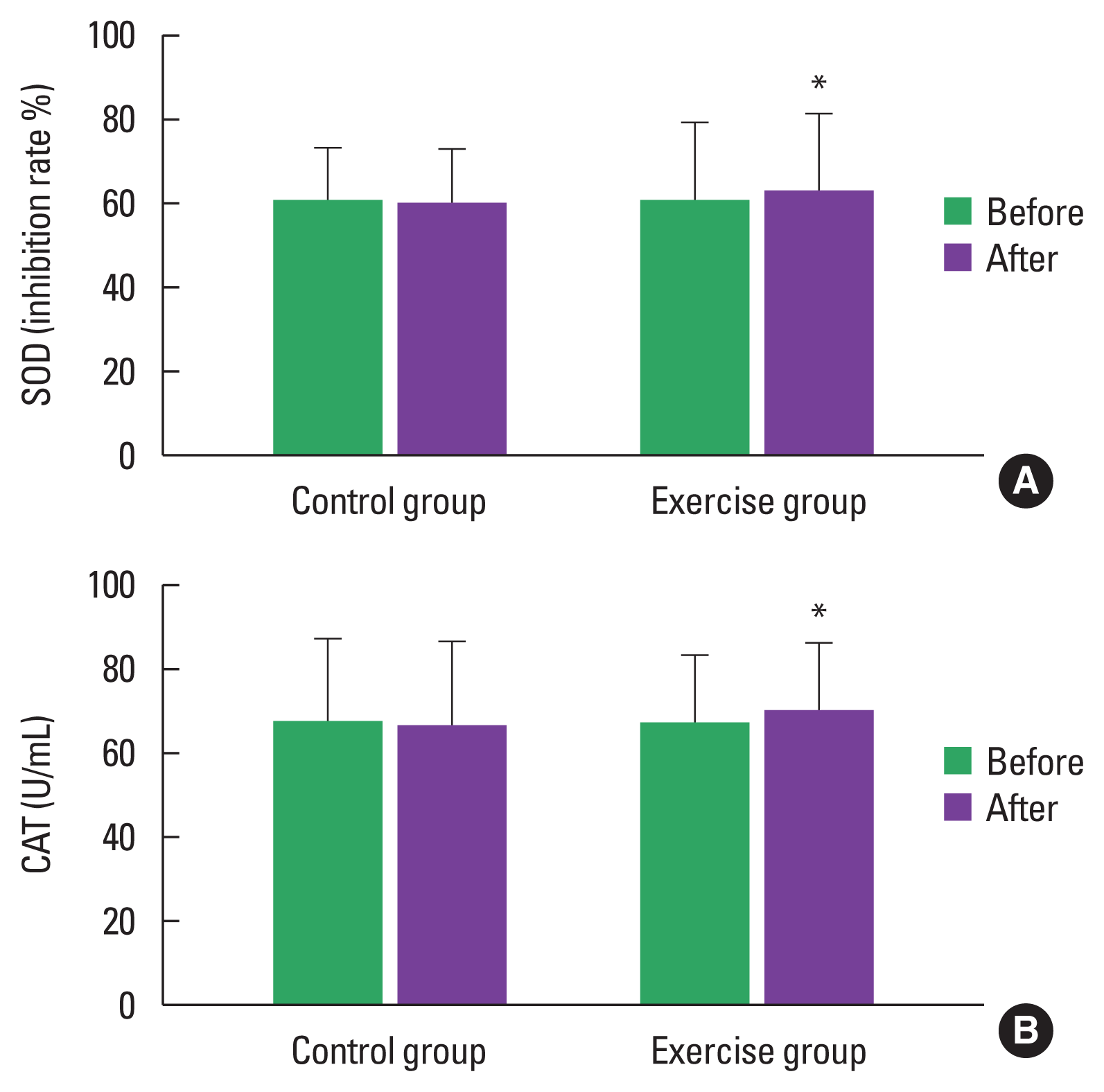
FBS and HbA1c were significantly decreased by 3.48% (P<0.05) and 2.51% (P<0.05), respectively, in the exercise group (Table 2; Fig. 3A, B). The presence of oxidative stress in patients with T2DM has been shown in the form of increased products of lipid peroxidation and altered antioxidant status. MDA results from lipid peroxidation of polyunsaturated fatty acids, and the degree of lipid peroxidation can be estimated by the amount of MDA in plasma or tissues. The oxidative stress parameters exhibited the following change when compared to baseline: MDA, a lipid peroxidation marker, drastically dropped by 8.7% (P<0.05) in the exercise group (Table 2, Fig. 4A). Antioxidants are substances which can prevent or slow down the damage to cells caused by free radicals such as SOD and CAT. The exercise group also presented greater antioxidant activity both in SOD and CAT, as evidenced by an increased percentage inhibition rate of SOD (60.37–62.82 inhibition rate %) (P<0.05) and increased amount of CAT (67.55–70.34 U/mL) (P<0.05) (Table 2; Fig. 5A, B).
The measurement of blood glucose and oxidative stress markers at before and after 12 weeks in control and exercise groups
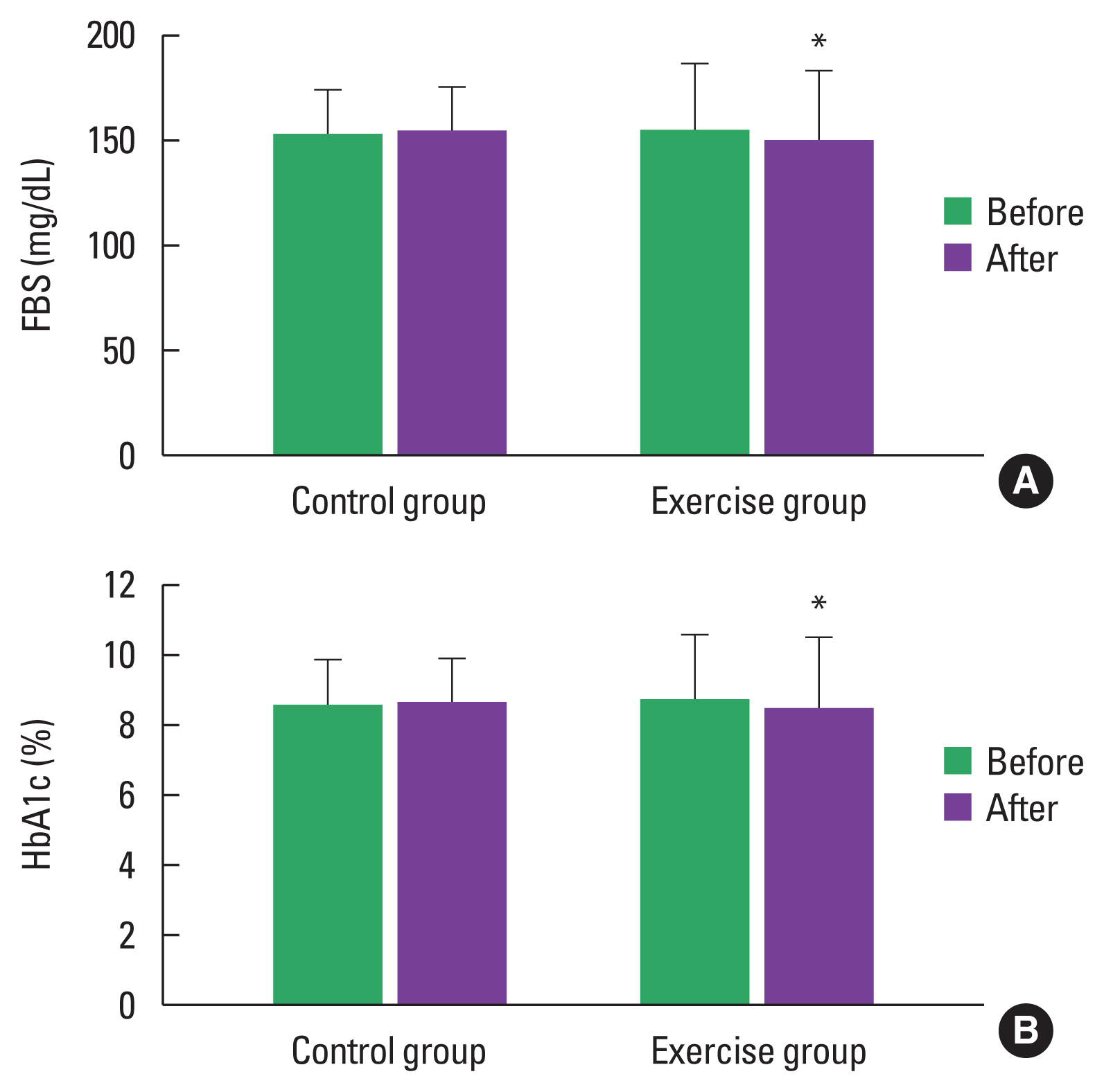
Fasting blood sugar (FBS) (A), glycated hemoglobin (HbA1c) (B) before and after 12 weeks in the control and exercise groups. Values are presented as mean±standard deviation. *P<0.05 to within-group comparison (before vs. after).

Malondialdehyde (MDA) (A) and fractional exhaled nitric oxide (FeNO) (B) before and after 12 weeks in the control and exercise groups. Values are presented as mean±standard deviation. *P<0.05 to within-group comparison (before vs. after).
Effects of EBRE with modified Thai yoga on pulmonary function in T2DM patients
Pulmonary function tests are used to quantify lung function, evaluate effectiveness, diagnose lung disease, and determine disability. The most widely employed pulmonary function test evaluates dynamic lung volume consisting of FEV1, FVC, FEV1/FVC, PEF, and FEF25%–75%. These variables reflect pulmonary abnormalities such as airflow limitation and restriction amid lung expansion. Pulmonary functions were exhibited as %predicted values (Table 3). The results revealed that T2DM in the exercise group significantly improved FEV1 (P<0.01), FVC (P<0.01), FEV1/FVC (P<0.05), PEF (P<0.05), and FEF25%–75% (P<0.05) compared to baseline. Whereas, there were no significant differences in the control group. These results imply that 12 weeks of exercise can improve both lung volume and airflow in T2DM patients.
Effects of EBRE with modified Thai yoga on respiratory muscle strength in T2DM patients
Respiratory muscle strength is an indicator of good respiratory system function. It can be assessed by measuring PImaxFRC, PImaxRV, and PEmax. PImaxFRC reflects the strength of the diaphragm, PImaxRV reflects the strength of the diaphragm and accessory inspiratory muscles, and PEmax reflects the strength of the expiratory muscles. The values of PImaxFRC, PImaxRV, and PEmax were significantly increased following 12 weeks of EBRE with modified Thai yoga compared to the control group (Table 4). The increased percentages were 7.3% (P<0.01), 16.1% (P<0.05), and 5.7% (P<0.01), respectively. This suggests that EBRE with modified Thai yoga improves respiratory muscle strength in patients with T2DM.
Effects of EBRE with modified Thai yoga on airway inflammation in T2DM patients
Exhaled NO is mainly produced by the endothelial cells of micro vessels in the airways and alveoli, with only a small portion produced by pulmonary vessels which can be assessed from the FeNO level. FeNO levels are elevated in the inflammatory condition. Subsequent to 12 weeks of exercise, FeNO level was significantly reduced by 17.2% (30.13±5.10 ppb vs. 24.95±6.41 ppb, P<0.05) compared to before experimentation. There was no noticeable difference in the control group (29.49±6.06 ppb vs. 30.32± 7.21 ppb) (Fig. 4B).
DISCUSSION
The evidence demonstrating the significant health benefits of EBRE with modified Thai yoga in T2DM patients led to the development of this study. T2DM patients exhibit elevated blood glucose levels and oxidative stress markers and reduced pulmonary function. This is the first study to evaluate the combination of EBRE and modified Thai yoga efficacy as an adjuvant therapy for reducing blood glucose levels, lowering oxidative stress, and enhancing antioxidant and pulmonary function in older T2DM patients.
The current study revealed substantial decreased levels of FBS and HbA1c in the exercise group who performed EBRE with modified Thai yoga. The crucial factor in lowering the probability of chronic DM complications was reported to be an improvement in glycemic control (Arslan et al., 2014). FBS and HbA1c in T2DM improved after 3 months of yoga and pranayama exercises (Balaji et al., 2011). EBRE twice a day, 5 days a week, for 12 weeks reduced FBS, HbA1c, postprandial glucose, and fasting insulin in T2DM patients (Park et al., 2016). In older persons with T2DM, significant reductions in FBS and HbA1c were reported following 16 weeks of home-based progressive resistance tube training (Ooi et al., 2021). Numerous studies have demonstrated that resistance training causes alterations in specific molecular signaling as well as physiological responses. Increased insulin sensitivity in T2DM patients following an exercise program was proposed (Schwingshackl et al., 2014). With the adjustment of the insulin-glucagon ratio, yoga training decreases insulin resistance, and increases insulin receptors and sensitivity resulting in shifts in the peak level of insulin to the left (Skarfors et al., 1987). Practicing yoga can improve glycemic control in those with diabetes by increasing insulin secretion and regenerating pancreas cells (Sahay, 2007). Additionally, yoga increases blood supply to the muscles, possibly enhancing the expression of insulin receptors on the muscles, leading to increased glucose uptake from the muscles and, therefore, decreasing blood sugar (Balaji et al., 2011). Furthermore, a decrease in blood glucose was associated with a decrease in oxidative stress and an increase in antioxidant activity in DM (Fiorentino et al., 2013).
Oxidative stress is evidenced by an increase in MDA and a decrease in antioxidants such as SOD, CAT, and glutathione (GSH) (Patil et al., 2014). In the current study, we found that doing EBRE with modified Thai yoga for 12 weeks significantly reduced MDA and increased SOD and CAT levels. Consistently, Patil et al. (2014) demonstrated that 3 months of yoga practice resulted in improvements in MDA levels as well as antioxidant levels of SOD, GSH, and vitamin C in elderly individuals with grade-I hypertension. They suggested that increased oxygen utilization from exercise causes excessive production of reactive oxygen species. Yoga may cause low oxygen consumption, which would reduce MDA levels. Another study showed that acute exercise reduces oxidative stress and increases liver antioxidant enzymes and muscular function (Kostić et al., 2009). Besides that, resistance training and high-intensity interval training for 12 weeks reduced MDA levels while increasing SOD in T2DM patients (Sudarsono et al., 2019). In untrained males, elastic resistance training and traditional resistance training enhanced SOD and GSH levels, while decreasing MDA levels (Kalvandi et al., 2019). Additionally, healthy males who performed elastic band resistance training 3 times per week on alternate days for 8 weeks demonstrated a reduction in MDA levels accompanied by increases in SOD, GSH, and total antioxidant capacity (Kalvandi et al., 2022). Moreover, in T2DM patients, 3 months of yoga practice enhanced antioxidant indicators (GSH and vitamin C) and decreased oxidative stress (MDA) (Hegde et al., 2011). Exercise training has been also shown to reduce plasma oxidation and lipid peroxidation, produce adaptive responses in endogenous antioxidants, and possibly have an impact on redox homeostasis and, consequently, redox-dependent adaptation mechanisms (Pittaluga et al., 2015).
EBRE with modified Thai yoga showed significant therapeutic effects on pulmonary function in older T2DM patients (FEV1, FVC, FEV1/FVC, PEF, and FEF25%–75%). Similarly, progressive aerobic and resistance training in T2DM were observed to enhance pulmonary function (FEV1 and FVC) following 12 weeks (Osho et al., 2012). In T2DM patients, an 8-week yoga program improved FVC, PEF, maximum voluntary ventilation, and diffusing lung capacity (Vishakha et al., 2020). In addition, elastic band resistance training combined with breathing techniques for 6 weeks can improve FEV1, FCV, and FEV1/FVC in elderly females (Kim et al., 2019). Moreover, in individuals with diabetic lung disease, adjunct yoga training enhanced FEV1, FVC, and FEV1/FVC (Balaji et al., 2019). The mechanisms responsible for the increase in pulmonary function following EBRE with modified Thai yoga may induce in increased respiratory muscle strength and chest wall mobilization - allowing for maximum breathing. Previously, Singh et al. (2012) suggested that the benefits of pranayama and yoga postures on pulmonary function are due to nonspecific bronchoprotective or bronchorelaxing effects. Additionally, yoga exercise increases blood oxygenation and reduces sympathetic activity (Bernardi et al., 2001). Therefore, a reduction in tracheobronchial smooth muscle tone may increase airway diameter leading to a reduction in airflow resistance, greater alveolar perfusion, and ultimately an improvement in pulmonary function (Soni et al., 2012). Throughout yoga training, there was increased air retention as well as consistently controlled inspiration and expiration. This causes maximum pulmonary inflation and deflation which increases pulmonary function (Mandanmohan et al., 2003).
The exercise group exhibited a significant increase in respiratory muscle strength (PImaxFRC, PImaxRV, and PEmax). The respiratory muscles will therefore become stronger resultant of exercising the diaphragm, abdominal muscles, and other respiratory muscles. These improvements support the findings of Yamamoto-Morimoto et al. (2019), who demonstrated a significant improvement in PImax post-yoga asana with pranayama exercise. What’s more, D’Souza and Avadhany (2014) reported significant improvements in PImax and PEmax following yoga exercise. Furthermore, exercise on the treadmill with elastic bands has been demonstrated to increase the strength of the respiratory muscles (Lima and Seo, 2011). The respiratory muscles were strengthened using diaphragmatic and abdominal breathing associated with yoga. Yoga allows the joints to move at different angles, enhancing muscle strength, and flexibility (Srinivasan, 2016), as well as promoting chest and abdominal expansion (Gonzalez-Alvarez et al., 2016), and increasing blood flow to the respiratory muscles (Posadzki and Parekh, 2009). Moreover, EBRE can also prolong the respiratory muscles’ endurance, especially in patients who utilize ventilators (Chang, 2020). Hence, EBRE with modified Thai yoga involves both muscle contractions, which are known to enhance the strength of skeletal muscles overall, and respiratory muscles - particularly the diaphragm as well as internal and external intercostal muscles.
FeNO measurement is a simple tool for assessing airway inflammation. Our findings demonstrate a decrease in FeNO level following a 12-week EBRE with modified Thai yoga. Yoga practice has been associated with reductions in systemic inflammation cytokine as indicated by lowering the levels of C-reactive protein, tumor necrosis factor-alpha (TNF-α), and interleukin 6 (IL-6) (Estevao, 2022). In small-scale industry workers, yoga training for 60 min, 6 times per week for over 12 weeks reduced IL-6, TNF-α, and C-reactive protein levels (Shete et al., 2017). Oxidative stress plays a crucial role in the pathogenesis of airway inflammation (Cho and Moon, 2010). The results of this study revealed a decrease in oxidative stress and an increase in antioxidants following EBRE with modified Thai yoga. Thus, the given exercise may influence several inflammation-mediating factors resulting in a decrease in airway inflammation, leading to improved dynamic lung function as shown by increasing the percentage prediction of FEV1, FVC, and FEF25%–75% in patients with T2DM. The recent study revealed that EBRE could reduce blood glucose, oxidative stress, and airway inflammation leading to improve pulmonary function. Hence, these worthy findings could be advised as a potential exercise program for elderly with T2DM.
The study has some limitations. Firstly, the effect of elastic resistance combined with yoga exercise on variables was only evaluated twice prior to and post intervention. The tendency to alter during intervention is unknown. For this reason, future studies should also evaluate these variables during training. Second, diabetes has a significant bearing on the functioning of the cardiovascular system. Nonetheless, this study lacked the effect of elastic resistance combined with yoga exercise on this system. Thus, further research could investigate the parameters of cardiovascular function such as lipid profiles, blood pressure, heart rate, and autonomic nerve system. Third, in this study, FeNo was employed to assess airway inflammation. To confirm that elastic resistance combined with yoga exercise is able to reduce inflammation, future studies ought to evaluate proinflammatory cytokines markers. Fourth, there were no data available on monitoring dietary intake in participants. This could have an impact on their antioxidant status. However, during the trial, the participants were asked and reminded not to change their eating behaviors. Finally, this study did not specify the consequences of elastic bands with unaccompanied. But previous studies have shown the benefits of both methods of exercise in patients with T2DM, leading to the combination of elastic bands and Thai yoga exercise into a new exercise program. Therefore, further study is required performing elastic bands and Thai yoga exercise in separately to provide more extensive data.
In conclusion, the current findings suggest that EBRE with modified Thai yoga can improve blood glucose, oxidative stress, antioxidants, pulmonary function, respiratory muscle strength, and airway inflammation in older T2DM patients. Thus, EBRE with modified Thai yoga could be recommended to the elderly with T2DM.
ACKNOWLEDGEMENTS
This research was supported by the Thailand Science Research and Innovation Fund and the University of Phayao (Grant No. FF64-UoE023 and FF66-RIM049). The participants’ contributions to the study are very much appreciated.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.