INTRODUCTION
Neuropathic pain is often pronounced as a shooting or burning pain, which is caused by damage affecting the central and peripheral nervous systems (Kumar et al., 2018). In general, peripheral neuropathic pain is subdivided into dysesthesia and allodynia according to symptoms and signs. Dysesthesia involves a disagreeable sensation such as a painful burning, prickling or aching feeling, and allodynia is defined as an unpleasant sensation that occurs with light touch. Sciatic nerve injury (SNI) is more closely related to allodynia than to dysesthesia (Mukai et al., 2019). Patients with neuropathic pain after SNI have suffered from poor quality of life due to anxiety, depression and severe pain (Bernetti et al., 2021). Therefore, many researchers have tried to find out mechanisms of neuropathic pain for several decades (Finnerup et al., 2021), but the mechanism is not yet clear.
According to previous studies on peripheral pain mechanisms reported until recently, it has been reported that Wnt signaling pathway is associated with neuropathic pain, classified into the canonical Wnt/β-catenin pathway and the noncanonical β-catenin-independent pathway (Itokazu et al., 2014). The canonical Wnt pathway results in an accumulation of β-catenin in the cytoplasm through binding several transmembrane receptors, including the frizzled receptor (FZ), low-density lipoprotein receptor 6 (LRP6) and low-density lipoprotein receptor 5 (LRP5), and stabilized β-catenin is translocated into the nucleus to activate transcription factors (Zhao and Yang, 2018), while the noncanonical pathway does not involve β-catenin as well as it not binding with LRP5 and LRP6 (Komiya and Habas, 2018).
In studies on central nerve degeneration and SNI, the canonical Wnt pathway has been known as an indicator of pain that increases inflammatory cytokines such as the nuclear factor kappa light chain enhancer of activated B cell (NF-kB), tumor necrosis factor-α (TNF-α), and interleukin-6 in the dorsal root ganglion (DRG) and the dorsal horn of the spinal cord (L’episcopo et al., 2011; Marchetti and Pluchino, 2013; Xu et al., 2018; Zhang et al., 2010). Dworkin et al. (2007) suggested that inhibition of Wnt protein expression through drug treatment and surgery resulted in a decrease in threshold of mechanical allodynia in about 30% of patients with neuropathic pain. However, these treatment methods have a large economic burden compared to their effectiveness (Bussa et al., 2021).
Regular aerobic exercise including walking, running and swimming has been used as one of the therapeutic strategies for motor and/or sensory recovery of damaged nerves in the field of the neuropathology and neurology (Sarikcioglu et al., 2009). Looking at some previous studies that confirmed the effect of exercise on neuropathic pain, it was reported that both forced and voluntary exercise not only increased the expression level of the glial cell-derived neurotrophic factor (GDNF) in the injured nerves but it also decreased the incidence of mechanical allodynia by 7% after spinal cord injury (Detloff et al., 2014) and peripheral nerve injury (López-Álvarez et al., 2015). Furthermore, Shen et al. (2013) reported that swimming exercise attenuated inflammation and peripheral neuropathic pain.
With these results found in previous studies, continuous physical activity is a positive effector closely related to neuropathic pain and mechanical allodynia after nervous system injury. However, research on exercise-regulated Wnt/β-catenin signaling at early phase regeneration of the injured sciatic nerve is still insufficient. Therefore, the purpose of this study focuses on investigating the effect of treadmill training on the pain-related Wnt/β-catenin signaling pathway in lumbar 4 to 5 DRG neurons at the early stage of regeneration after SNI.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (150–160 g, 6 weeks old) were used in this experiment. They were randomly divided into seven groups: the normal control, sedentary groups for 3-, 7-, 14-day post crush (dpc), and exercise group for 3, 7, 14 dpc (n=10 in each group). Animals were maintained at a constant room temperature of 22°C and 60% of humidity under 12/12-hr light-dark cycle. They were accepted to eat commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. This experiment obtained approval by the Ethics Committee of Jeju National University (2018-0028).
Sciatic nerve injury
The rats in the injury group were anesthetized using an animal inhalation narcosis control (Jeungdo Bio & Plant, Seoul, Korea). First, the rats were placed into the chamber with a 2%–2.5% concentration of isoflurane for anesthesia and then 1.5%–1.8% concentration for maintenance during SNI. The left sciatic nerve was crushed with a pair of forceps held tightly for 1 min and 30 sec at intervals (Seo et al., 2006). After surgery, anesthetized animals were then placed on a heating pad maintained at 37°C, and then they were put in their cages for resting. All rats were sacrificed 3, 7, and 14 days later.
Treadmill exercise
All rats used in the experiment were adapted to treadmill walking exercise for a week before the study began. Excluding the control group, sciatic nerve surgery was performed in the exercise and sedentary groups. After resting for 2 days, all rats in the exercise group performed a low-intensity walking exercise on the treadmill device (Jeungdo Bio & Plant) on 8 m/min for 20 min with no inclination during experiment duration (3, 7, and 14 dpc).
Western blot analysis
Lumbar 4 to 5 DRG neurons play a role in transmitting the sensory signal into the central nervous system. The dissected DRG were rinsed with phosphate-buffered saline, and lysed in Triton lysis buffer. The nucleus and cytoplasm were separated by nuclear extraction buffer and cytosol extraction buffer. Denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4°C. Protein (20 μg) was used for Western blot analysis using anti-mouse Wnt3a (1:1,000, Cell Signaling Biotechnology, Danvers, MA, USA), anti-rabbit phosphorylated LRP6 (1:1,000, Cell Signaling Biotechnology), anti-mouse LRP6 (1:1,000, Cell Signaling Biotechnology), anti-mouse β-catenin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti- mouse β-actin (1:2000, Santa Cruz Biotechnology) antibodies. For the secondary antibody, Horseradish peroxidase-conjugated anti- mouse or anti-rabbit IgG antibodies (1:1,000, GeneTex Inc., Irvine, CA, USA) were used. The blotting proteins were detected by using Westar ECL substrates (Cyanagen, Bologna, Italy). Detected band intensity was analyzed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Paw withdrawal test
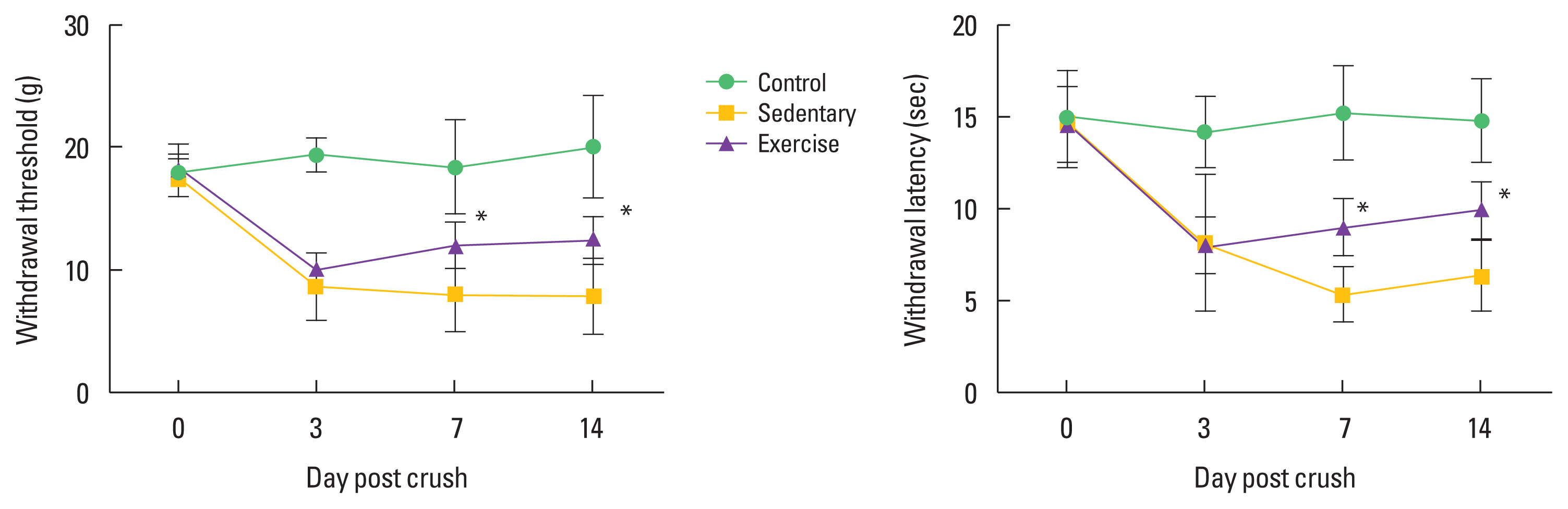
For assessment of mechanical allodynia, rats were placed on a clear individual box with holes and the animals were allowed to adapt for 30 min before the test. The von Frey filament (BIO-EVF4, Bioseb, Vitrolles, France), plastic hairs of calibrated diameters, was applied to the plantar surface of the left hind paw in a series of ascending forces (Yalcin et al., 2014). All rats were always tested for 3 times and the time and intensity of hind paw withdrawal was measured. The filaments that were the most often used are 0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, and 10 g. The von Frey test was performed before the injury (0 day), 3, 7, and 14 dpc.
Statistical analysis
All the data is presented as a mean±standard error. Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test. The significance level was set at P<0.05. All data analysis and graphs were performed by using Prism 6 (GraphPad, La Jolla, CA, USA).
RESULTS
Treadmill exercise improved mechanical allodynia after SNI
To confirm the effect of treadmill exercise on peripheral pain-like behavior after SNI, we analyzed the time-dependent effect on latency for hind paw withdrawal using the von Frey test device. As shown in Fig. 1, exercise groups at 7 and 14 dpc significantly ameliorated mechanical allodynia compared to the sedentary groups, but there was no significant difference between exercise and sedentary group at 3 dpc.
Treadmill exercise decreased Wnt3a and p-LRP6 levels in the DRG neurons after SNI
Wnt3a and LRP6 have been known as a pain marker of the injured nervous system. To investigate the change of pain-related proteins after SNI, we analyzed the time-dependent effect of treadmill exercise on Wnt3a and LRP6 in DRG neurons after SNI. As shown in Fig. 2, Wnt3a was significantly decreased in treadmill exercise groups at 3, 7, and 14 dpc compared to sedentary groups. LRP6 protein showed no significant difference between sedentary and exercise groups at all time points, while phosphorylated p-LRP6 in exercise groups turned out to be significantly lower than those in sedentary groups at 3 and 14 dpc.
Treadmill exercise regulates translocation of β-catenin to nucleus of DRG neurons after SNI
To examine translocation of β-catenin from the cytoplasm to the nucleus of DRG neurons after SNI, we analyzed alterations of β-catenin in lumbar 4 to 5 DRG neurons and then confirmed a quantitative measure of transported β-catenin in cytoplasm and nucleus of DRG neurons. As shown in Fig. 3, β-catenin was significantly decreased in exercise groups at 3 and 14 dpc compared to sedentary groups. In the cytoplasm of DRG neurons, β-catenin did not indicate a significant difference between sedentary and exercise groups at all time points, while translocation of β-catenin to the nucleus of neurons further decreased in exercise groups 14 dpc compared to those in sedentary groups.
DISCUSSION
Although many previous studies reported that aerobic and anaerobic exercises are effective in reducing neuropathic pain after SNI, these findings do not suggest a specific mechanism of exercise for mechanical allodynia in the early stage of axonal nerve regeneration (Kami et al., 2017). Therefore, this study investigated pain-related biochemical and behavioral changes through exercise in the early stage of regeneration.
Neuropathic pain in nervous system disorders is a pain condition caused by abnormal sensations called dysesthesia and allodynia. Peripheral nerve damage including SNI usually produces allodynia rather than dysesthesia (Bourquin et al., 2006). Yalcin et al. (2014) represented that paw withdrawal threshold in the von Frey test would be the most reliable method of mechanical allodynia induced after SNI. Thus, we applied mechanical allodynia assessment using the von Frey test device, and confirmed that treadmill exercise dramatically decreased the symptoms of mechanical allodynia at all time points compared to sedentary groups. In previous studies on neuropathic pain-like behavior after peripheral nerve injury, Farzad et al. (2018) suggested that regular exercise for 4 weeks resulted in a beneficial effect on neuropathic pain symptoms after peripheral nerve injury through activation of irisin and glutamic acid decarboxylase-65 protein. But Whitehead et al. (2017) reported conflicting results that acute wheel-running activity did not alter low-intensity mechanical allodynia in the sciatic chronic constriction injury model. The different results between the previous studies and this study are thought to be due to differences in the SNI method, regenerative phase, duration of exercise application, exercise type and exercise intensity.
For decade, many researches on the role of Wnt signaling into the nervous system have made extraordinary advancement with respect to our understanding of embryonic brain development, mammalian neurogenesis, and tumorigenesis (Girardi and Le Grand 2018; Hu et al., 2019). The Wnt protein has been well known as an indicator that underlies the pathogenesis of neuropathic pain in rodents. Central or peripheral nervous injury leads to a rapid-onset induction of Wnt in the primary sensory neurons (Zhang et al., 2013). Wnt co-receptor LRP6 also is dramatically increased in association with activated Wnt signaling (Simonetti et al., 2014). In our study, the prototype canonical Wnt3a and LRP6 in the exercise group showed a tendency to decrease continuously from 3 days until 14 days after SNI. Inceoglu et al. (2015) confirmed that Wnt inhibitors improved mechanical allodynia and thermal hyperalgesia in neuropathic pain models as well as swimming exercise decreased on expression of Wnt3a-LRP proteins in Wallerian degeneration condition. These previous studies and present findings imply that regular exercise can be a positive effector on the blocking of neuropathic pain-related protein expression after SNI.
In addition to induction of Wnt and LRP6, β-catenin also is a multifunctional protein that activates Wnt signals in rat model with neuropathic pain (Peng et al., 2019). We established that treadmill exercise downregulated translocation of β-catenin to nucleus of DRG neurons after SNI. During neuropathic pain stage, β-catenin is mainly activated in neurons by binding of Wnt and FZ receptors, and it is translocated from cytoplasm to nucleus for activating T-cell factor/lymphoid enhancer factor series transcription factors (Wisniewska et al., 2010). In other words, nuclear translocation of β-catenin in DRG neurons can aggravate mechanical allodynia induced by SNI. Thus, present study that treadmill exercise suppressed nuclear accumulation of β-catenin in DRG cells to regulate mechanical allodynia was consistent with some previous studies showing that the Wnt/β-catenin should be blocked to improve neurotrophic pain (Zhang et al., 2013), suggesting that exercise may be a potential regulator for attenuating neuropathic pain.
Given these findings reported in the present study, regular treadmill exercise would be effective in controlling neuropathic pain including mechanical allodynia. In conclusion, the present finding provided critical evidence that physical exercise might regulate neuropathic pain after peripheral nerve injury through delayed Wnt/β-catenin signaling pathway in DRG neurons.